2023 Pharma IssueIn the March issue of Review of Optometry, experts provide exciting updates on incoming dry eye treatments, oral steroid prescribing protocols and advancements in presbyopia management. Plus, learn how to fight insurance denials for new meds. Check out the other articles featured in this issue:
|
Part 2 is below. Part 1 can be found here.
Often, what’s found at clinical presentation results in needing additional laboratory testing and/or imaging studies. In Part 1 of this series last month, we explored how to implement these procedures in your practice, who should be ordering the testing, along with the challenges of doing so. Here, in the second part of our two-part series on laboratory and imaging studies, a few scenarios are explored where these tests can be used in the optometrist’s office. Several cases are presented where these studies played a crucial role in diagnosis, with all of the following scenarios presenting regularly to the optometrist. Understanding the role these tests play in patient management is imperative for the practicing clinician.
Anterior Segment: Inflammation
From an anterior segment perspective, some of the more common instances where lab studies are needed are in situations when the patient presents to the office with anterior segment inflammation, either with or without a previously diagnosed systemic etiology. Particularly challenging are the situations where no underlying diagnosis exists.
The patient who presents with inflammation of the anterior segment unfolds regularly in many clinics. As usual, the examination begins with a thorough clinical history. In many cases, the history is positive for some type of extraneous source that is contributing to the inflammation, such as contact lens overwear or ocular surface disease. Naturally, reducing inflammation in these cases occurs by treating the source of the problem, such as with inflammatory dry eye.
But where do you go when there are no clinically evident sources of inflammation from the history or physical exam and the spectre of autoimmune diseases is raised? This is often when lab studies become warranted. Often, these patients will present to the clinic with cases of episcleritis or anterior uveitis.
| Click here to read Part 1 of this two-part series on laboratory testing and imaging in optometric practice. |
Episcleritis is often induced by external sources, as mentioned, and eliminating the underlying etiology can result in resolution of the episcleritis. However, it is estimated that upwards of 25% to 35% of patients with episcleritis have an underlying systemic disorder, which can be infectious or inflammatory in nature.1 Diffuse episcleritis is more common than nodular episcleritis. A variety of systemic diseases are associated with episcleritis, including the autoimmune disorders lupus, rheumatoid arthritis, ulcerative colitis and Crohn’s disease. Infections are also a possible cause, such as Lyme disease and herpes zoster virus.2,3
Given the relatively common incidence of episcleritis, not all cases need a systemic work-up. A good rule to follow is to consider work-ups when the condition is chronic, recalcitrant or bilateral, without obvious exogenous sources or if a systemic condition is suspected based upon review of symptoms. In many situations, we treat these patients empirically with topical steroids and/or oral NSAIDs.
Anterior uveitis follows the same line of action, with initial empirical topical therapy with steroids and cycloplegics in many cases. In general, though, recurrent, recalcitrant, bilateral and/or granulomatous anterior uveitis increases the likelihood of systemic comorbidities as outlined above.
A word of caution here: When treating uveitis empirically, it is imperative to treat the condition aggressively with topical cycloplegics and steroids. Undertreatment can result in rebound uveitis, which may ultimately be misdiagnosed as a recurrent case, as opposed to simply undertreated inflammation. Aggressive treatment often includes atropine and a strong topical steroid with good penetration into the anterior chamber, such as Pred Forte (prednisolone acetate, Allergan) or Durezol (difluprednate, Novartis). Occasionally, subconjunctival steroid injections or oral steroid treatment (in addition to the aforementioned topical agents) are needed to wrest control of the inflammation. If, after aggressive treatment, the uveitis flares periodically or is recalcitrant, then an underlying systemic disease should be considered, especially if the initial presentation was bilateral and granulomatous.
Also keep in mind that autoimmune diseases usually take years to manifest in their full form, and can often create signs and symptoms in individuals in the 20- to 50-year-old range. As such, it is not uncommon for early lab studies to come back negative or initially be inconclusive. For this reason, exercise caution in ordering lab studies on all patients who initially present with anterior segment inflammation; the presentation and history are critical in initially determining when and when not studies should be ordered, as outlined above.
Sarcoidosis is a systemwide inflammatory condition characterized by pulmonary infiltration and granuloma formation. It is associated with elevated angiotensin converting enzyme (ACE) and a Westergren erythrocyte sedimentation rate (ESR) level. ACE is elevated in about 60% to 90% of active sarcoidosis, but consider that it may also be elevated in cases of histoplasmosis, toxoplasmosis and tuberculosis.4,5 Pulmonary infiltration with granulomatous tissue is seen on plain film X-rays and computed tomography (CT) imaging of the chest, which are part and parcel of the work-up of a patient suspected to have sarcoidosis. Both ACE and ESR results are nonspecific markers of inflammation. In other words, they may be elevated in a variety of inflammatory conditions, not just sarcoidosis.
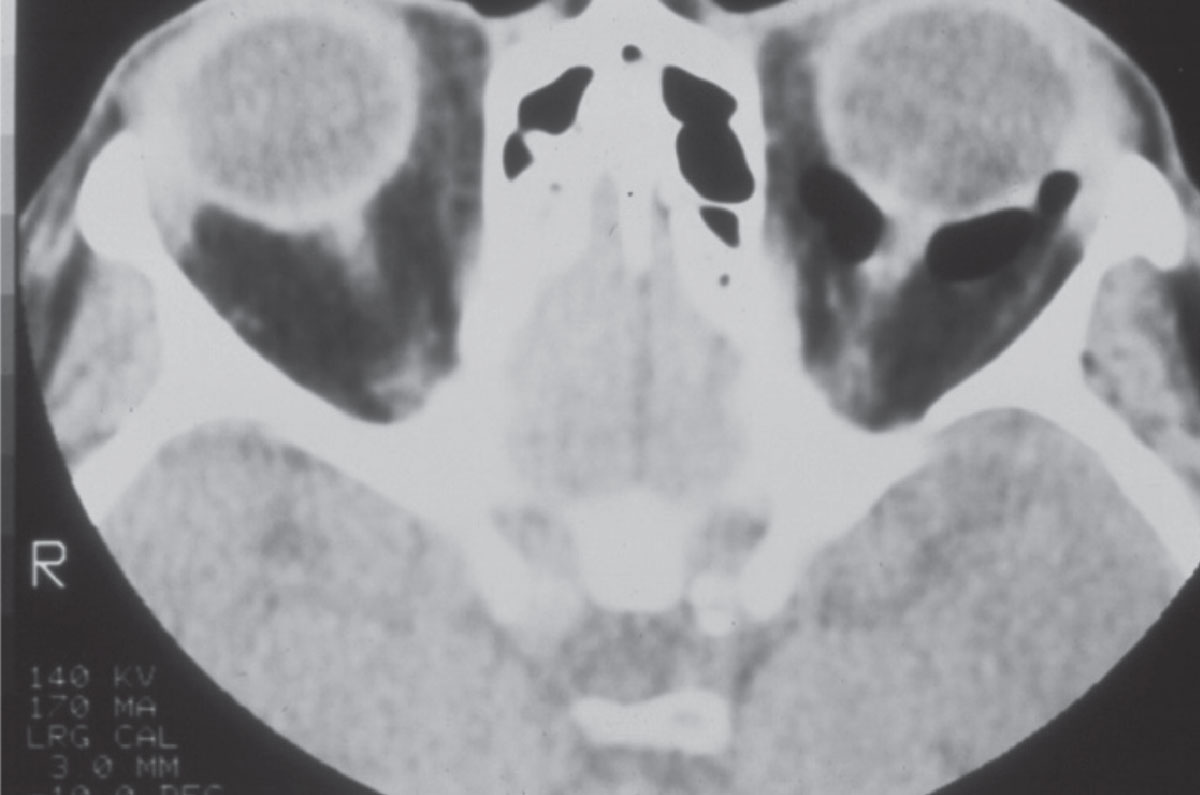 |
|
Fig. 1. A CT image of the orbits in a patient with a left orbital floor fracture. Note the image void behind the left eye; this is air, and the retrobulbar orbit is contiguous with the maxillary sinus beneath due to the fracture. Click image to enlarge. |
Rheumatoid arthritis and lupus are autoimmune disorders with elevated ESR and C-reactive protein levels (CRP). Note that these two indices are also simply a measure of current inflammatory activity and are not specific to these autoimmune disorders. Rheumatoid factors include various antibodies that are elevated in about 70% to 90% of patients with rheumatoid disease. Anti-nuclear antibodies (ANA) are antibodies that ultimately attack self cell nuclei. Interestingly, low or negligible levels of ANA typically rule out lupus, whereas elevated levels can indicate the presence of lupus, rheumatoid, scleroderma and Sjögren’s. Anti-citrullinated protein (peptide) antibodies (ACPA) are also elevated in the majority of patients with rheumatoid arthritis.6
HLA-B27 is a specific type of protein found on white cells and is elevated in certain autoimmune diseases, such as ankylosing spondylitis, reactive arthritis and psoriatic arthritis. Most human leukocytic antigens are protective, but HLA –B27 can cause the opposite effect. Because the immune system uses proteins encoded by HLA to essentially determine which proteins are self and which are foreign, the HLA complex plays a protective role. However, certain HLA typing is associated with autoimmune disorders. In particular, HLA-B27 presents self and non self-antigenic proteins to T-cells, resulting in an autoimmune reaction.7
While the patient demographics are different between autoimmune diseases, there are many similarities that exist amongst them. However, as the name implies, juvenile rheumatoid arthritis (JRA) affects individuals younger than those with non-JRA rheumatoid. Also, ankylosing spondylitis patients have certain physical characteristics (lumbar spine abnormalities seen on plain film) that for example, a patient with systemic lupus erythematosus would not have. Delving deep into the history of these patients is critical in offering a target lab approach to facilitate a diagnosis.
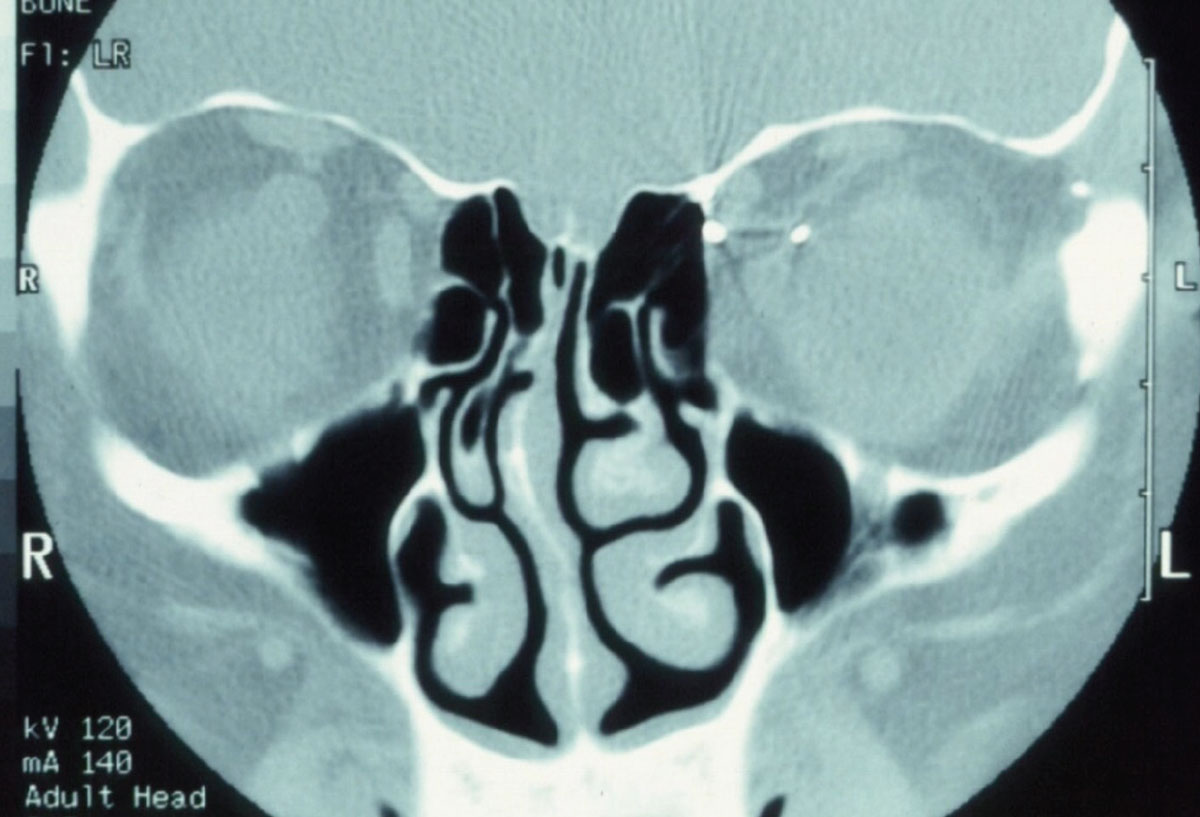 |
|
Fig. 2. Pictured here is a metallic foreign body in the left orbit. Consider that MRI physics employs a powerful magnet to obtain images. In cases where a metallic foreign body is suspected in the orbit or globe, CT imaging is warranted so as to not violently dislodge the foreign body. Click image to enlarge. |
Typically, I’ll consider ordering an initial panel of labs for patients presenting with inflammation that is bilateral, granulomatous, recurrent or recalcitrant despite proper therapy. Which labs are ordered are generally tied to patient history with a suspected autoimmune disorder. Patients without a diagnosis of autoimmune disease are a challenge, as their systemic symptoms may simply be due to physical activity rather than an underlying condition. Typically, these initial labs include a complete blood count (CBC) with differential (indicative of infectious vs. inflammatory, etiology), ESR, CRP, rheumatoid factors ACPA and ANA. I also include a fluorescent treponemal antibody absorption test and venereal disease research lab test or rapid plasma regain test, as syphilis being the great masquerader, it can induce many of the ocular signs of inflammation that we see. In addition to these tests, I will add ACE levels and plain chest films (or CT imaging) if sarcoid or tuberculosis is suspected through the presence of persistent cough or malaise. Conversely, if the patient is younger, and I suspect ankylosing spondylitis or Reiter’s, as characterized by anterior uveitis and urethritis, I’ll add HLA-B27 and lumbar plain films or CTs.
Ultimately, the underlying systemic condition will be managed by the primary care physician, internal medicine or rheumatologist, but the OD is sometimes the first provider to initiate the work-up of a patient with undiagnosed systemic conditions manifesting as anterior segment inflammation. Coordinating with the PCP and/or rheumatologist can ultimately lead to the systemic diagnosis and consequently ensure appropriate treatment of these underlying conditions.
Posterior Segment Findings: Retina
Following are some conditions that ODs should feel comfortable ordering lab studies for, especially in emergent situations, such as with arteritic ischemic optic neuropathy.
One of the most common indications I see in clinic for ordering lab studies are retinal hemorrhages. As we know, the retina has a dual blood supply: one from the choroid and the other, more anterior circulation from the central retinal arteries.
Hemorrhages due to abnormalities of the anterior retinal circulation can result from arterial hemorrhages, venular hemorrhages or hemorrhages of the retinal capillary bed. Many instances present multiple hemorrhaging sources. Typically, retinal arterial hemorrhages result in ‘flame-shaped’ hemorrhages due to their anterior location in the nerve fiber layer. These are often the result of hypertension, Valsalva maneuvers and other diseases that affect the arterial tree, such as red blood cell anemias.
Conversely, diabetes mellitus typically induces hemorrhages in its early stages, based either in the retinal capillary bed or early venous segments. These tend to be dot and blot hemorrhages, owing to their deeper location in the retina.
Retinal venular hemorrhages typically appear as larger blot hemorrhages, sometimes associated with venular dilation. Though retinal hemorrhages can be caused by numerous systemic etiologies, the heart of lab studies associated with retinal hemorrhaging in non-diabetic patients is the CBC test. It’s estimated that about 70% of hematologic conditions can be diagnosed with a CBC.8
Retinal arterial plaques are usually embolic in nature and can originate from degenerating atheromas, the heart or the carotid artery system. Patients with acute retinal emboli, either in the form of a branch retinal artery occlusion or a central retinal artery occlusion require urgent neuroimaging to assess stroke risk and cerebral ischemia but may also need further lab studies for dyslipidemia, aberrant glucose levels and red and white cell abnormalities.
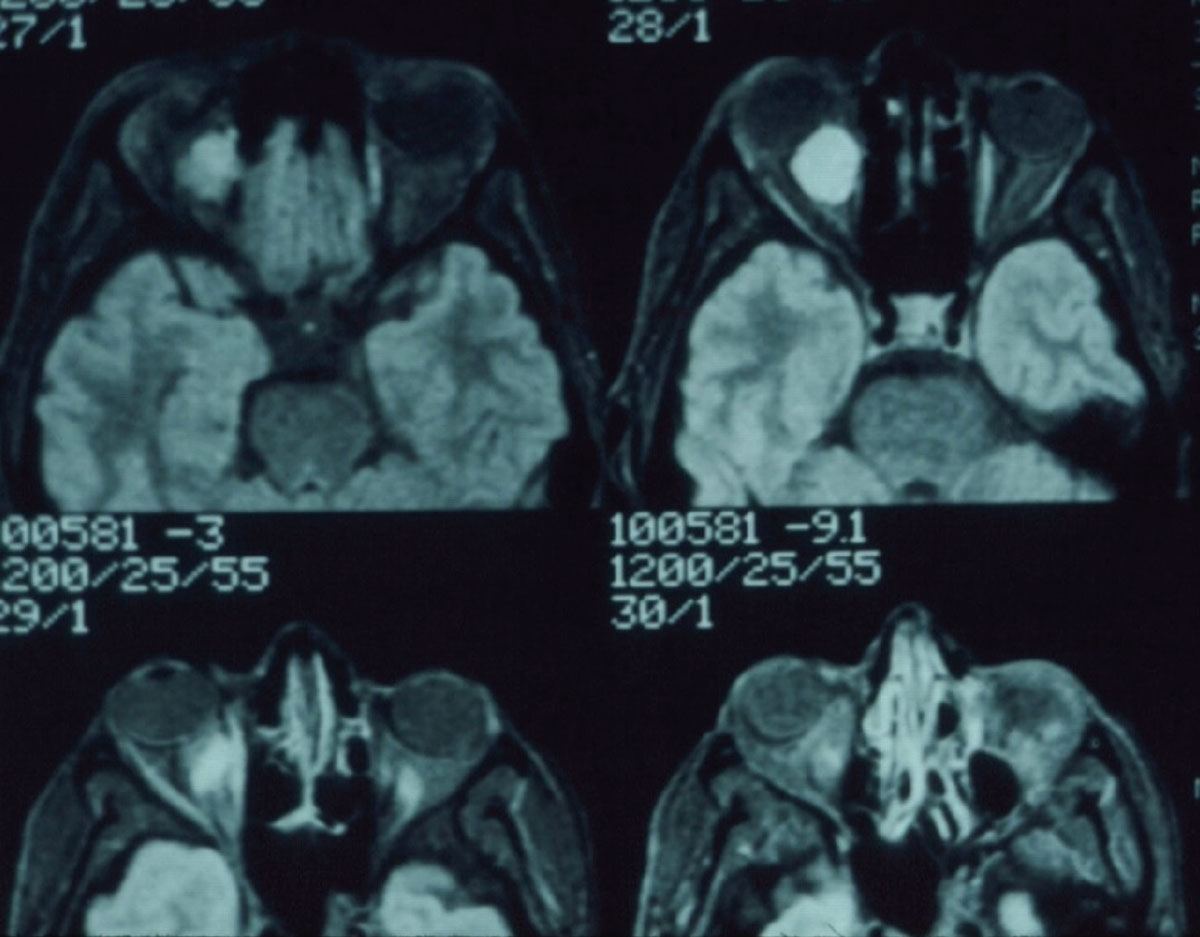 |
|
Fig. 3. Note the mass in the retrobulbar space of the right orbit. Click image to enlarge. |
The important point here is that retinal hemorrhaging can be caused by numerous conditions and the associated lab studies are directly tied to what the suspected underlying condition is. The overlap of atherosclerotic cardiovascular disease and many other vascular disease presentations in the retinal arterial, capillary and venular systems is the basis for evaluations of those entities that are associated with the development of this disease. Namely, these include diabetes, hypertension, hyperlipidemia, smoking and obesity.
Accordingly, lipid panels are an integral part of the work-up of many patients with retinal vasculopathies that imply atherosclerotic cardiovascular disease origins. These panels will include total cholesterol, triglycerides, HDL and LDL levels. New research suggests that stratified lipid panels provide a better picture of the systemic risk of dyslipidemia, with subtyping of HDL and LDL particles in particular.9
Of course, diabetes plays a large role in retinopathy in developed countries, and in lab studies, glycosylated hemoglobin is a strong indicator of glucose control and future risk of diabetic retinopathy development.
Posterior Segment Findings: Optic Nerve
Optic neuropathies originate from several different etiologies, but the non-glaucomatous optic neuropathies, specifically anterior ischemic optic neuropathy (AION) and non-arteritic anterior ischemic optic neuropathy (NAION), present frequently in patient populations over the age of 50.
Arteritic ischemic optic neuropathy (giant cell arteritis) is a medical emergency. This typically affects patients over the age of 65 and is characterized by sudden loss of vision, disc edema, dyschromatopsia and visual field loss, temporal head pain, jaw claudication and malaise. It is associated with inflammation of medium-sized arteries, including those in the distribution of the external carotid artery. The signs and symptoms of arteritic AION still remain an important criteria in impetus to begin the initial work-up of a patient suspected of having arteritic ischemic optic neuropathy.10
From a lab perspective, patients suspected of having arteritic AION need immediate laboratory investigations, including (primarily) an ESR and CRP. Elevated ESR and CRP levels, with a CRP greater than 2.45, are diagnostic of arteritic AION along with the aforementioned clinical findings. Treatment with intravenous or oral steroids is indicated to prevent similar ischemia in the fellow eye and the diagnosis is confirmed with temporal artery biopsy. Temporal artery ultrasonography is becoming more of a diagnostic tool in lieu of biopsy, especially in light of its easy accessibility and noninvasive methodology.11
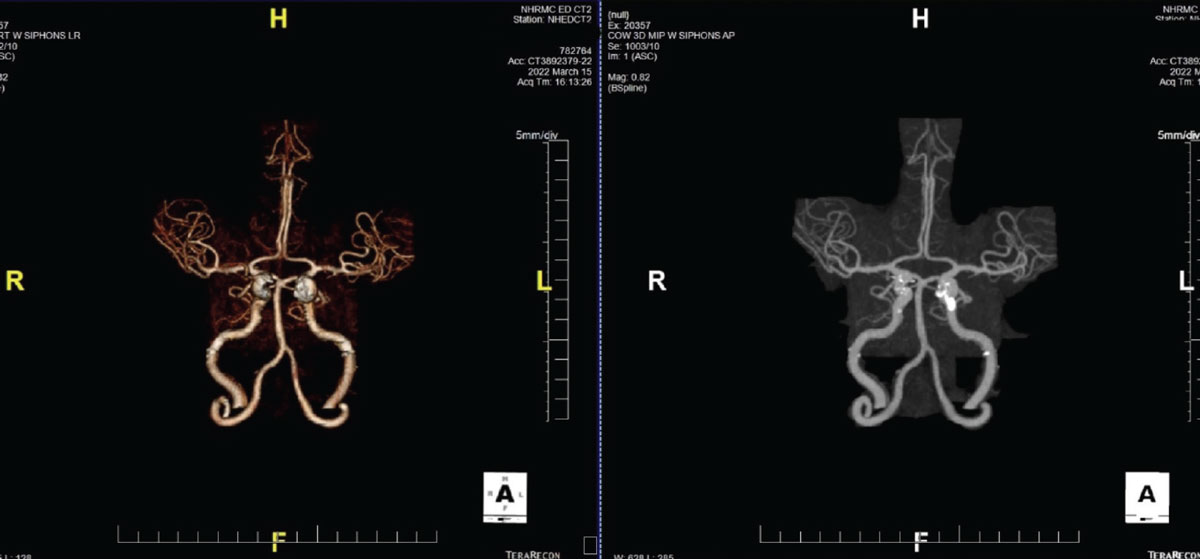 |
|
Fig. 4. A CTA demonstrating significant plaque formation in a patient with transient visual obscurations. Note the calcification of the L>R internal carotid arteries. Click image to enlarge. |
NAION is also characterized by unilateral visual field loss, more often noted upon awakening, and typically affects somewhat younger patients than AION. This too is an ischemic infarct of the optic nerve, but not associated with inflammation of the branches of the external carotid artery. This is an infarct involving the small vessels of the optic nerve head and it is related ultimately to the same risk factors that are associated with the development of atherosclerotic cardiovascular disease. Prominent risk factors include diabetes, hypertension, hyperlipidemia, smoking, obesity and a sedentary lifestyle. Another risk factor is the use of PDE5 inhibitors, such as Cialis (tadalafil, Eli Lilly) and Viagra (sildenafil, Pfizer), more so in patients with a small, crowded disc, which in and of itself can be a risk factor—the so-called “disc at risk.”
Management of NAION is an internal medicine driven intervention, aimed at tightly controlling the aforementioned risk factors. Thus, lab studies for lipids and glucose are an integral part of managing these patients long-term.
Computed Tomography (CT)
This imaging uses electromagnetic radiation to generate images; this is the same type of energy used in plain film X-rays and therefore has limits to the amount of exposure a patient should have to this type of radiation.
CT imaging from an ophthalmic perspective is mainly useful to identify problems involving bone, the interface of bone and air and fresh hemorrhaging. Its soft tissue resolution is not nearly as high as with MRI.
The technology, however, is quite useful in orbital fracture evaluations, as the interface of bone (hyperintense on CT imaging) and air (dark) in the paranasal sinuses is quite indicative of bony abnormalities (Figure 1). Likewise, evaluation of the paranasal air sinuses can best be done by CT imaging. CT imaging additionally has value in cases where intraorbital or intraocular foreign bodies are suspected (Figure 2).
As previously outlined in Part 1 of this article in the February 2023 issue, CT imaging in the emergency department is routinely performed on admitted unconscious patients for the evaluation of intracerebral hemorrhaging, due to fresh blood showing up readily on CT imaging. This imaging modality is also generally more accessible than MRI on short notice, but does have its own limitations, especially when looking at soft tissue detail.
Occasionally, we will use CT imaging to evaluate intracranial contents when there is suspected a mass or a mass effect. While subtle detail may not be present, gross structural changes can readily be seen, thereby offering an alternative to MRI in the initial evaluation of certain patients. The same holds true for orbital disease, especially cases of recent onset proptosis. While extraocular muscles are generally better visualized on MRI, a quick CT scan of the orbits can shed light onto the possibility of Graves’ disease or orbital myositis or space occupying lesion (Figure 3).
With the increased usage of CT angiography (CTA), CT imaging has seen a resurgence in the number of cases being ordered from our office in the context of neuroimaging, especially if a vascular problem is suspected. CTA does a fantastic job of highlighting vasculature throughout the body (Figure 4). It is routinely performed in evaluation of patients with chest pain and coronary artery disease, as its images are outstanding in identifying vascular disease.
From an ophthalmic perspective, CTA is useful in patients who present with transient visual obscurations due to suspected carotid, vertebral or cerebral vascular disease. While the brain parenchyma is not visualized in CTA in detail, the presence or absence of significant vascular abnormalities is evident. These patients may still need MR imaging or intravascular catheter angiography, but in cases where the patient needs to be imaged quickly, this is a suitable alternative to MRI and MRA imaging.
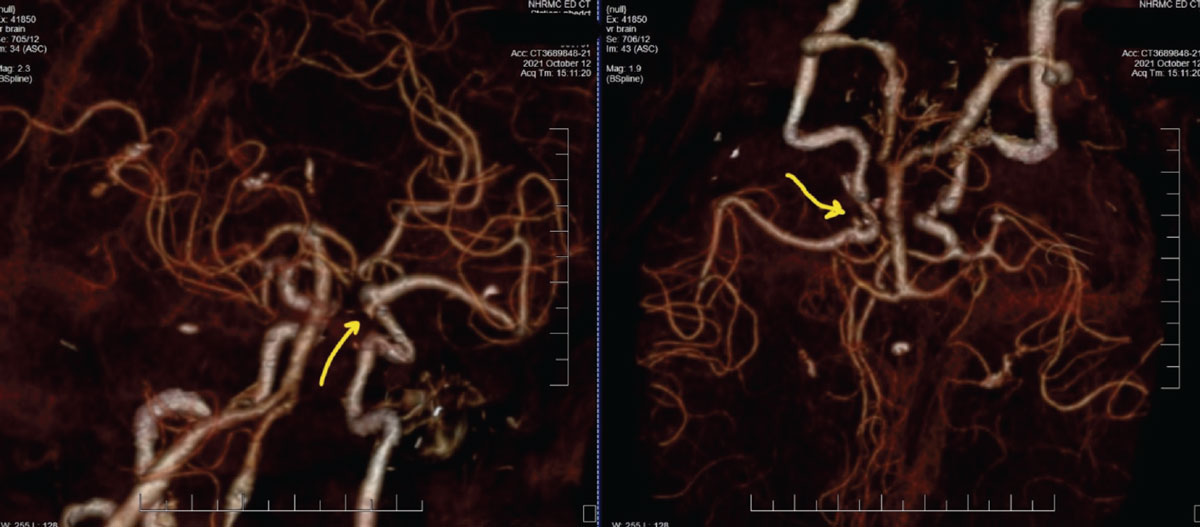
Case 1. Here is one example showing the practical use in ordering a CTA. An 87-year-old female patient presented with painful and acute CNIII palsy (Figure 5). The pupil was involved. She had a complex medical history, including hypertension and hypercholesterolemia of many years’ duration. Patients with these conditions are considered medical emergencies, as rupture of cerebral aneurysms at the junction of the internal carotid artery and the posterior communicating artery can prove fatal, or at best, debilitating.
 |
|
Fig. 5. These CTA images of the patient described show two different rotational views of the carotid, vertebral and basilar arteries, along with distributions of the anterior and middle cerebral arteries. The yellow arrows are directing to the aneurysm at the junction of the internal carotid artery and posterior communicating artery, which is the source of the third nerve palsy. Click image to enlarge. |
Given the urgency of the situation and the reality that, in our area, obtaining MRI imaging can prove more delayed than CTA, this patient was sent to the emergency department (ED), accompanied by a call from myself to the attending ED physician, outlining the findings and requesting a CTA. Without the relevant information presented to the ED physician, there is a higher likelihood that neuroimaging without angiography (CTA or magnetic resonance angiography (MRA)) would have been obtained instead, thus delaying the vascular diagnosis even further. This was discussed at length in Part 1 of this series.
Given the findings, the patient was then sent to neurosurgery and ultimately underwent coiling of the aneurysm. Included is the radiology report of the patient in Case 1 for reference (Figure 6).
 |
|
Fig. 6. This is a portion of the radiologist’s report in the Case 1. Most of these reports discuss the findings seen in the imaging ordered. Toward the end of the report, a conclusion is made of the radiologic findings, often accompanied by suggestions for further evaluation. Note that, for the radiologist to make further suggestions, an integral part of their interpretation and plan involves the information you initially conveyed to them. Click image to enlarge. |
Do note that when neuroimaging is ordered by any physician, including an optometrist, the radiology report accompanies the scan results, wherein the neuroradiologist makes a diagnosis based on the structural details seen on the imaging. While the OD is not necessarily interpreting the scans themselves, it is still good practice to read or examine them anyway to become more familiar with these images. The radiologist, however, is making the diagnosis. It then becomes incumbent upon the OD to act on that information in the appropriate fashion, which often involves referring the patient to the appropriate subspecialist.
Magnetic Resonance Imaging (MRI)
This imaging from an ophthalmic perspective is very useful in evaluating neuro-ophthalmic problems, orbital problems and soft tissue subtleties. MRI uses the basic principle of the polarity of water molecules being placed in a strong magnetic field; each tissue in the body has varying amounts of water and the tissue density differences seen on MRI is related to the amount of water in the tissue. Paramagnetic contrast media is often employed to highlight adjacent tissues.
MRI is very much software-driven and various imaging techniques are obtained through software manipulation such as fluid-attenuated inversion recovery (FLAIR), apparent diffusion coefficient (ADC) and diffusion weighting. For example, FLAIR is very useful in identifying demyelinating disease in the white matter adjacent to the ventricles, whereas ADC mapping and diffusion weighting are important in determining the chronicity of cerebral ischemia. These imaging techniques do not need to be specified when you order an MRI; rather, their use is determined by the radiologist, driven to a significant degree by the clinical findings you relay to them. Each imaging technique is geared to highlighting certain structures, and the radiologist uses those techniques to facilitate their diagnosis, similar to our use of various OCT image techniques.
The number of indications for MRI in eye care is quite large and they generally center around orbital and neuro-ophthalmic diseases. These include, but are not limited to, evaluation of patients with retinal emboli of acute nature, specifically looking for cerebral ischemia and the consequent risk of debilitating stroke, incidences of suspected papilledema due to elevated intracranial pressure, afferent visual system field defects (either acute or chronic), acute efferent cranial neuropathies involving CN III, IV and VI, Chiari malformations, a variety of orbital disease and numerous other conditions. Its major advantage is its capability to differentiate soft tissue and visualization of the brain parenchyma. Entire textbooks are available that demonstrate the various indications of MRI in eye care if you are interested in delving further into the topic.
Case 2. Here is an example demonstrating why an OD may request MRI. A 69-year-old Caucasian female presented to the office with complaints of severe headache followed by a short episode (about one minute) of dysphasia which occurred five days earlier. She had a history of diagnosed chronic migraine, but this particular episode was more severe than typical presentation, thus prompting her visit to the office. In-office evaluation did not demonstrate clinical findings associated with acute stroke at the time of visit. However, her history warranted neuroimaging.
 |
| Fig. 7. This is the typical radiology report you will see when ordering neuroimaging. In this case, both and MRI and an MRA were ordered. This first page is the report pertaining to the MRI. The MRA was entirely normal. Note here that as the findings are clearly laid out, it is incumbent upon the ordering provider to understand what these findings mean and ultimately to develop a plan related to these findings. Given these are consistent with small vessel ischemic disease, referral to internal medicine is warranted to mitigate the risks of continued atherosclerosis. While the initial symptoms initiated the MRI and MRA ordering, fortunately the patient did not require emergent or urgent treatment. Click image to enlarge. |
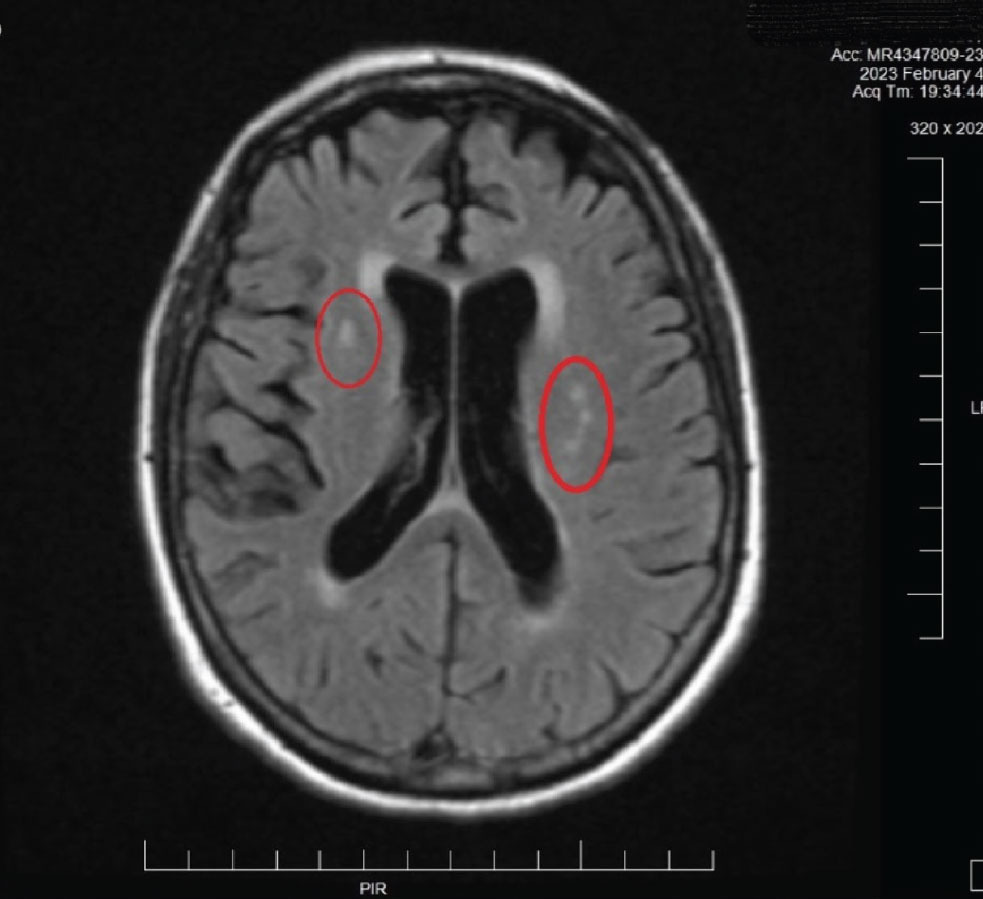
An urgent MRI/MRA was ordered (Figure 7). Based upon the radiologist’s interpretation of the scan, the patient was found to have no evidence of stroke or any significant issue requiring hospitalization. The only findings on MRI were areas consistent with chronic small vessel disease, consistent with her age and is atherosclerotic in nature. The patient was ultimately sent to internal medicine in order to manage the underlying atherosclerosis (Figure 8). The MRA report was normal, as was, essentially, the MRI. Further investigation for carotid and coronary disease is underway.
 |
|
Fig. 8. This MRI image is from the patient described in Case 2. Note the hyperreflective areas in the periventricular white matter. These are consistent with age-related small vessel occlusive disease. Also note the fine brain parenchymal images obtained with MRI. This imaging method is preferred over CT imaging of the brain in many cases for this reason: resolution of fine detail of soft tissues. Click image to enlarge. |
MRA can be obtained at the same appointment as MRI and highlights cervical and cerebral arteries. This is useful in visualizing the carotid arteries, vertebral arteries, the circle of Willis and the major cerebral arteries. MRA is often obtained at the same time as MR imaging in the evaluation of patients with impending embolic stroke or suspected ischemic stroke.
Case 3. Here is another case involving MRI. A 76-year-old Caucasian male presented with transient visual obscurations. There was a distinct lack of symptoms related to acute stroke (e.g., hemiplegia, hemiparesis), but the patient’s age, presenting and medical history increased the likelihood that he was in fact demonstrating symptoms of cerebral ischemia.
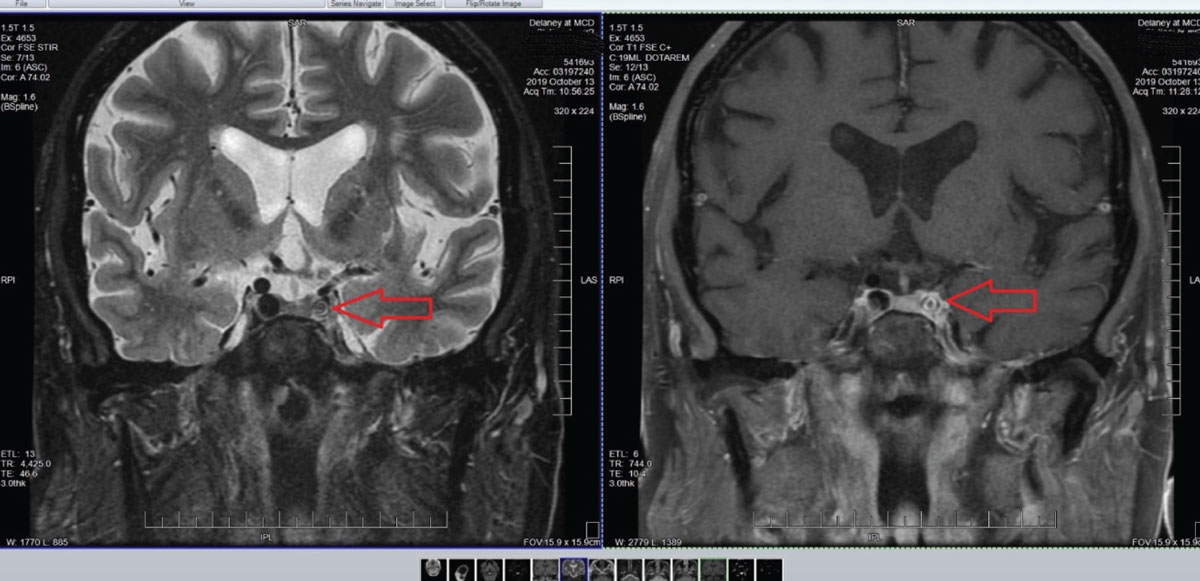
Accordingly, an MRI/MRA was ordered and findings included atherosclerosis (Figure 9). The presence of atherosclerosis of cerebral arteries in a patient with transient visual obscurations and without embolic plaque presence is managed by medical or surgical intervention, depending on the age and health of the patient and location of the atherosclerosis. Ultimately, this patient was deemed not fit to be a surgical candidate. Accordingly, internal medicine management of the atherosclerosis was undertaken to mitigate the risks of acute stroke.
 |
|
Fig. 9. Note in these images the marked stenosis of the internal carotid artery in the left cavernous sinus. Not surprisingly, other adjacent angiographic images demonstrated sclerosis of the middle and posterior cerebral arteries on the ipsilateral side and focal cerebral ischemia consistent with his chief complaint. Click image to enlarge. |
 |
|
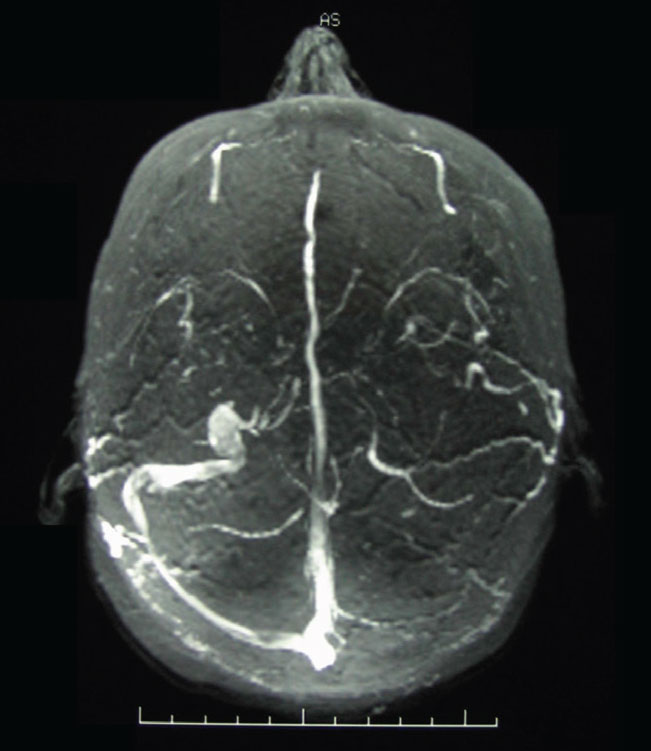
Fig. 10. An MRV image of transverse dural venous sinus stenosis in a patient with elevated intracranial pressure. The dural venous sinuses in MRV imaging are hyperintense; note the asymmetry of the transverse sinuses right vs. left. Click image to enlarge. |
Magnetic resonance venography (MRV), along with MRI, is an integral part of the work-up of patients with clinical evidence suggestive of elevated intracranial pressure without gross CT or MR findings of abnormalities in cases when we suspect idiopathic intracranial hypertension (IIH). Upwards of 90% of patients with IIH have stenosis of the transverse sinuses (Figure 10)12. When IIH is suspected, it is imperative to include an MRV at the same sitting as the MRI, as the dural venous sinuses will not be visible on MRI nor on MRA imaging.
Takeaways
From anterior to posterior segment, there is no shortage of instances that warrant an OD’s ability to call for further labs or imaging. While there are only three cases presented here, there exist many more than could be outlined. Though not all scenarios where neuroimaging is needed can be specified in one column, this piece should give ODs the essentials they need to carry with them when evaluating patients with underlying systemic conditions with ocular side effects.
Dr. Fanelli is in private practice in North Carolina and is the founder and director of the Cape Fear Eye Institute in Wilmington, NC. He is chairman of the EyeSki Optometric Conference and the CE in Italy/Europe Conference. He is an adjunct faculty member of PCO, Western U and UAB School of Optometry. He is on advisory boards for Heidelberg Engineering and Glaukos.
1. Associated diseases and diagnostic evaluation. American Academy of Ophthalmology. www.aao.org/focalpointssnippetdetail.aspx?id=fb9ffdf5-028a-40e4-9281-7a6a3b2319aa. Accessed February 1, 2023. 2. Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology. 1999;106(4):729-31. 3. Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, et al. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 2012;119(1):43-50. 4. Ezzie ME, Crouser ED. Considering an infectious etiology of sarcoidosis. Clin Dermatol. 2007;25(3):259-66. 5. Ang T, Juniat V, Selva D. Autoimmune markers in screening for orbital inflammatory disease. Eye (Lond). April 19, 2022. [Epub ahead of print]. 6. Toes RE, van der Woude D. ACPA (anti-citrullinated protein antibodies) and rheumatoid arthritis. Acta Reumatol Port. 2011;36(3):205-7. 7. Akkoç N, Yarkan H, Kenar G, et al. Ankylosing spondylitis: HLA-B*27-positive vs. HLA-B*27-negative disease. Curr Rheumatol Rep. 2017;26:19. 8. Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005;80(7):923-36. 9. Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013;62(8):732-9. 10. Hayreh SS. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4(5):251-63. 11. Luqmani R, Lee E, Singh S, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Access. 2016;20(90):1-238. 12. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418-24. |


