 |
When It’s Not Amblyopia: The Differential of Functional vs. Pathological Vision Loss
A clear understanding of the differences is key for effective patient management.
By Sherry J. Bass, OD, and Daniella Rutner, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: July 15, 2024
Expiration Date: July 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists who want to learn about the impact this system has on practice.
Educational Objectives: After completing this activity, participants should be better able to:
Differentiate between functional and pathological vision loss in clinical practice.
Effectively identify the AOA criteria that support amblyopia.
Recognize what factors can contribute to a misdiagnosis of amblyopia.
Determine when supplemental testing in amblyopia is necessary.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Bass has no financial disclosures. Dr. Rutner has received grant support from the Hoffman Foundation Grant and is a speaker for Apply EBP. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #128731 and course ID 92045-GO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
A patient with unilateral or bilateral vision loss can be a challenge for the eyecare practitioner. Is there a functional reason or disease process causing the loss of vision? While most times the reason is quite apparent, there are times when the diagnosis is not so obvious. If a practitioner diagnoses disease and there is a functional issue, that is not going to adversely affect the patient’s vision or life. However, if the reverse conclusion is made and the patient is diagnosed with a functional problem, e.g., amblyopia but there is an underlying disease process, the result can have significant effects on a patient’s vision and even life.
The misdiagnosis of amblyopia is costly. For the patient, it can mean wasted time pursuing treatment for a functional problem when they may have a condition that can result in permanent loss of vision and in some cases even death. For the eyecare practitioner, it can mean loss of reputation and career.
This article will review the American Optometric Association (AOA) Guidelines for the criteria that support amblyopia (amblyogenic factors) and will then cover case presentations that exemplify how the practitioner can best differentiate functional vision loss from pathological vision loss.1
 |
|
Click table to enlarge. |
AOA Guidelines
Amblyopia consists of more than reduced visual acuity and has a constellation of symptoms and clinical findings including increased difficulty with crowding effects, abnormal spatial distortions, unsteady and inaccurate or eccentric monocular fixation, poor tracking ability, reduced contrast sensitivity and inaccurate accommodative response.1-5 Functional amblyopia can be a result of form deprivation, (e.g., cataract, ptosis, etc.), constant unilateral strabismus and amblyogenic refractive error either high isoametropic or anisometropic (Table 1).1,6
The key is assessment of the patient. A careful history is critical. Age of onset of the condition is also important. While amblyopia can still develop up to six to eight years of age, if the child presents with sudden worsening of visual acuity; after this age, a thorough clinical evaluation must be performed to rule out other causes of vision loss.1 Visual acuity needs to be assessed based on the child’s age and ability to perform the test. When Snellen visual acuity testing is not feasible, tests of forced preferential looking (e.g., Cardiff Cards, Teller Acuity Cards) or matching, (Lea Symbols, HOTV chart) in young children may be an alternative.7
Testing visual acuity through a neutral density filter may be helpful in ruling out amblyopia. The visual acuity will usually be the same in amblyopia; whereas if the vision drops significantly, a disease process may be suspect. Refractive error assessment should be performed under noncycloplegic and cycloplegic conditions. Retinoscopy can be a valuable tool to give clues to ocular health (like keratoconus with scissor reflex or detecting media opacity) in addition to dioptric refractive value.8 While tropicamide can be used as a cycloplegic agent, in cases of strabismus and/or latent hyperopia, cyclopentolate is the drug of choice for controlling accommodation to assess refractive error.9
 |
|
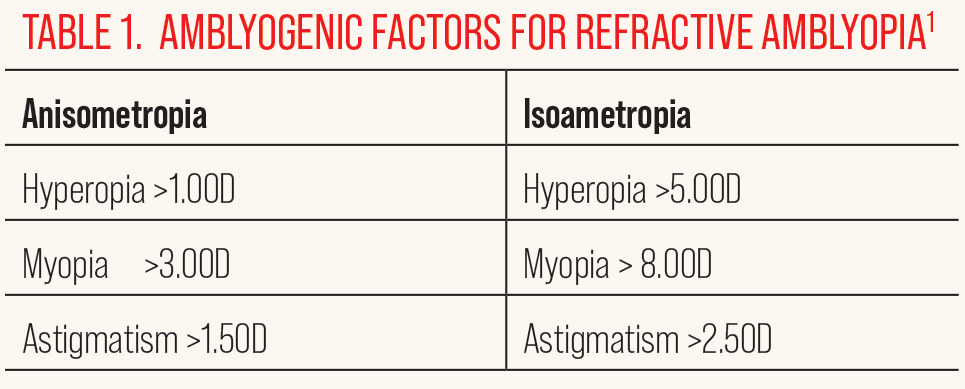
Fig. 1. Fundus photography reveals what appears to be a smaller optic nerve head in the left eye, suspicious for optic disc hypoplasia. Click image to enlarge. |
Monocular fixation can be an adjunct cause of reduced acuity in patients with amblyopia. Fixation assessment can be performed by monocular visuoscopy, with the calibrated target in the ophthalmoscope. More recently, studies have reported using optical coherence tomography (OCT) to assess eccentric fixation in children.10 Oculomotor deviation should be carefully assessed objectively. This should include a slow cover test to allow the fixating eye to pick up fixation; be mindful of both vertical and horizontal deviations. Small angle deviations, not clinically visible to cover test, may be revealed with ancillary sensorimotor fusion testing such as no stereopsis on random dot stereopsis and a failure to make a compensatory vergence movement with a 4^ prism. Normal stereopsis in a patient over eight years of age with recent onset best-corrected visual acuity (BCVA) loss should be suspect for disease.
Ocular health assessment should be carefully performed to ensure that there are no abnormalities. Under standard pupillary light testing, look for evidence of any pupillary abnormality.11,12 Amblyopia is a condition affecting form sense, not light sense, so pupil responses should be normal. Color vision testing is an underused test that is easy to perform and can signify inherited retinal and optic nerve disease. Color vision in amblyopia is normal, so a patient with bilateral reduced vision and reduced color vision is a red flag for a possible inherited retinal disease such as cone dystrophy. Careful slit lamp examination is imperative to assess the ocular corneal surface for any pathology and corneal topography should be assessed in cases where scissoring of retinoscope images was detected, or in cases of prominent corneal nerves and/or when a Fleischer ring is detected on the corneal surface— these may be signs of early keratoconus. Dilation should be performed, and a careful assessment of the lens should be conducted to rule out any lenticonus or subluxation. Subluxation can typically be viewed through a dilated pupil with Bruckner reflex on ophthalmoscopy, which may pick up subtle subluxations.13 Careful assessment of the macula and optic nerve is necessary when ruling out disease. Attention to the vasculature is also important since attenuated arterioles in a young individual could be a sign of retinitis pigmentosa. Unilateral hemorrhage and exudation in a young individual, especially a male, could signify Coats’ disease.
 |
|
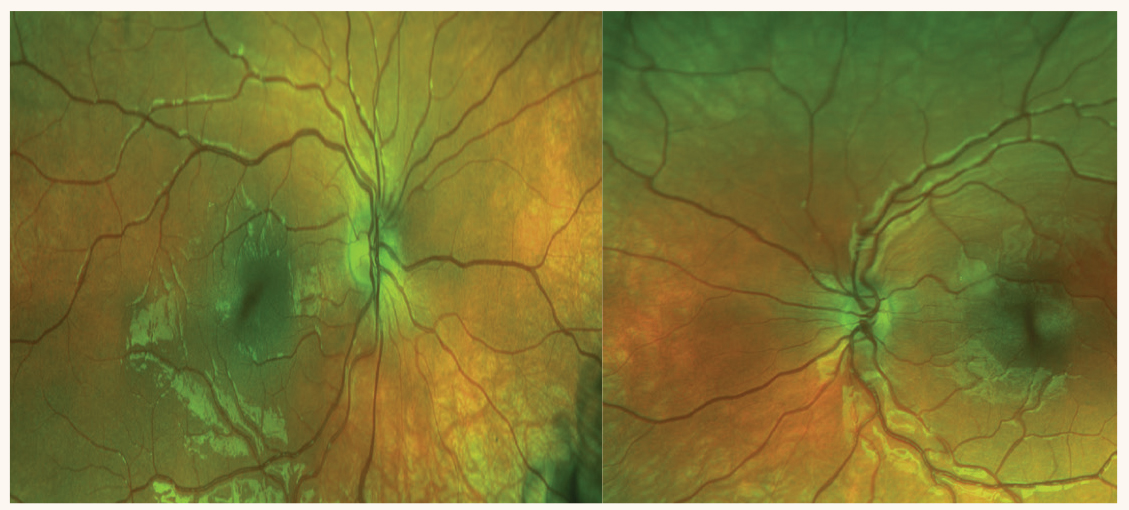
Fig. 2. OCT of the optic nerve head and RNFL on two visits. The patient was not too cooperative on the first visit (left) for the OS but improved on the second visit (right). Both optic nerve heads are normal and the RNFL is full, ruling out optic disc hypoplasia OS. The disc area OD and OS is also similar. Click image to enlarge. |
Technology and Differential Diagnosis
Supplemental testing in amblyopia should be ordered when there are no amblyogenic factors associated with the decrease in vision as previously described. Testing includes static visual field testing, OCT of the retina and optic nerve fiber layers, fluorescein angiography and fundus photography, including fundus autofluorescence imaging for inherited retinal diseases that affect the outer retina, such as retinitis pigmentosa (RP), Stargardt’s disease and cone dystrophy.1,13 Referral for electrodiagnostic testing such as visual evoked potential (VEP) and electroretinography (ERG), electro-oculography and/or neuroimaging are other tests that are important in ruling out retinal and visual pathway disease.1,13
Misdiagnosis of amblyopia is often the result of the failure to use or to refer for many of these available technologies. For example, many practitioners rely on confrontation visual fields (CVF) using fingers as a sufficient screener for visual field defects. Unfortunately, CVF using gross targets will only demonstrate abnormalities in cases of absolute field loss, such as in stroke, and end-stage glaucoma and inherited retinal degenerations. Relative field loss will be more difficult to detect. In the case of unilateral visual acuity loss due to an early space occupying lesion affecting the visual pathways, some form of static perimetry will be sensitive enough to pick up an early defect. VEP testing allows for objective testing of visual function and can be performed using patterned and non-patterned (flash) stimulation. Amblyopia is a disease of form sense, hence responses to pattern VEPs are reduced in amplitude and delayed, depending on the degree of amblyopia. However, responses to flash stimulation should be normal. If not, then the practitioner should suspect visual pathway disease. In young patients, space occupying lesions must be ruled out and neuroimaging may be necessary. ERGs are ordered when hereditary retinal diseases are suspected, such as RP, X-linked juvenile retinoschisis, cone dystrophy and Stargardt’s disease.
 |
|
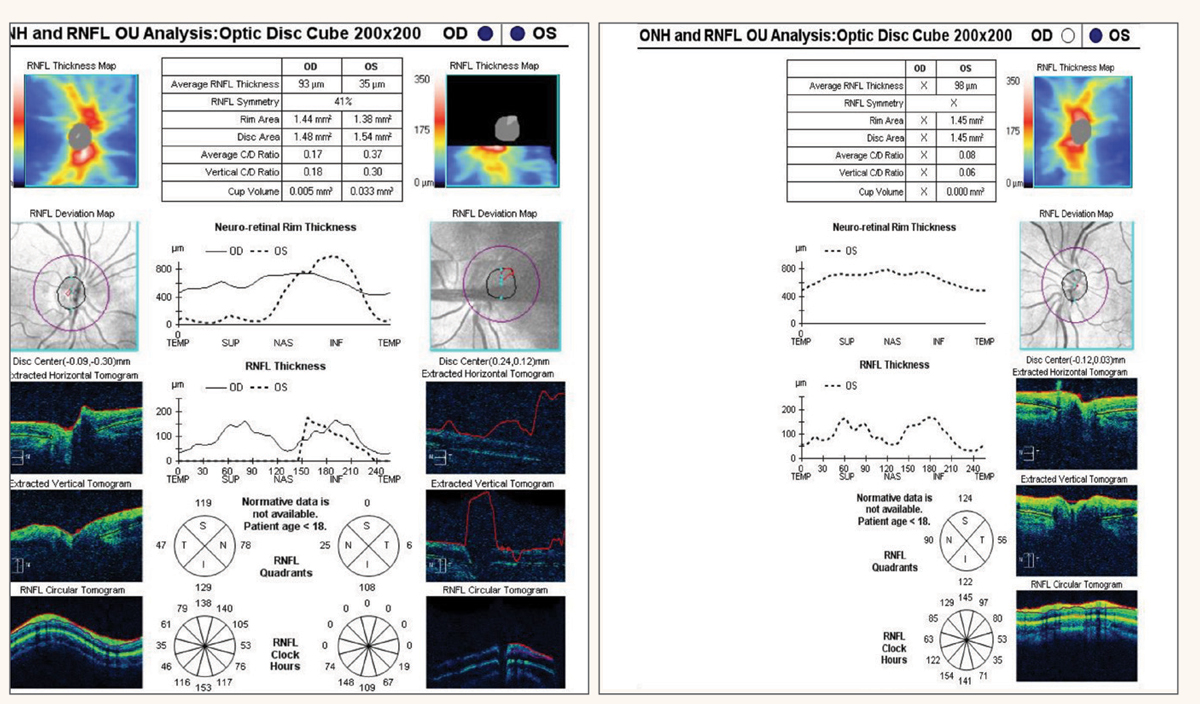
Fig. 3. Pentacam images OD and OS in 2021 (3a, left). Images in 2023 (3b, right). Both show a high degree of astigmatism, increasing in the OD, but regular corneas. The patient had been diagnosed with refractive amblyopia due to the high astigmatism but was correctable to 20/20 and 20/25 with GP contact lenses. This was not refractive amblyopia since the VA was correctable. Photo: Elianna Sharvit, OD. Click image to enlarge. |
Misdiagnosed Ocular Diseases
A consistent number of ocular disease conditions have been misdiagnosed as amblyopia, especially in young children, based on the referrals we have received. These conditions include, but are not limited to:
- Keratoconus
- Lens anomalies
- Inherited retinal disease, including inherited macular diseases
- Stargardt’s disease
- RP and its related syndromes
- X-linked juvenile retinoschisis
- Cone dystrophy
- Coats’ disease (unilateral vascular anomaly often seen in young males)
- Congenital optic nerve head anomalies and inherited optic nerve disease
- Dominant optic atrophy
- Optic nerve hypoplasia
- Juvenile glaucoma
- Visual pathway disease, including undiagnosed space occupying lesions
The following cases demonstrate how the proper use of technologies helped to differentiate functional from pathological vision loss.
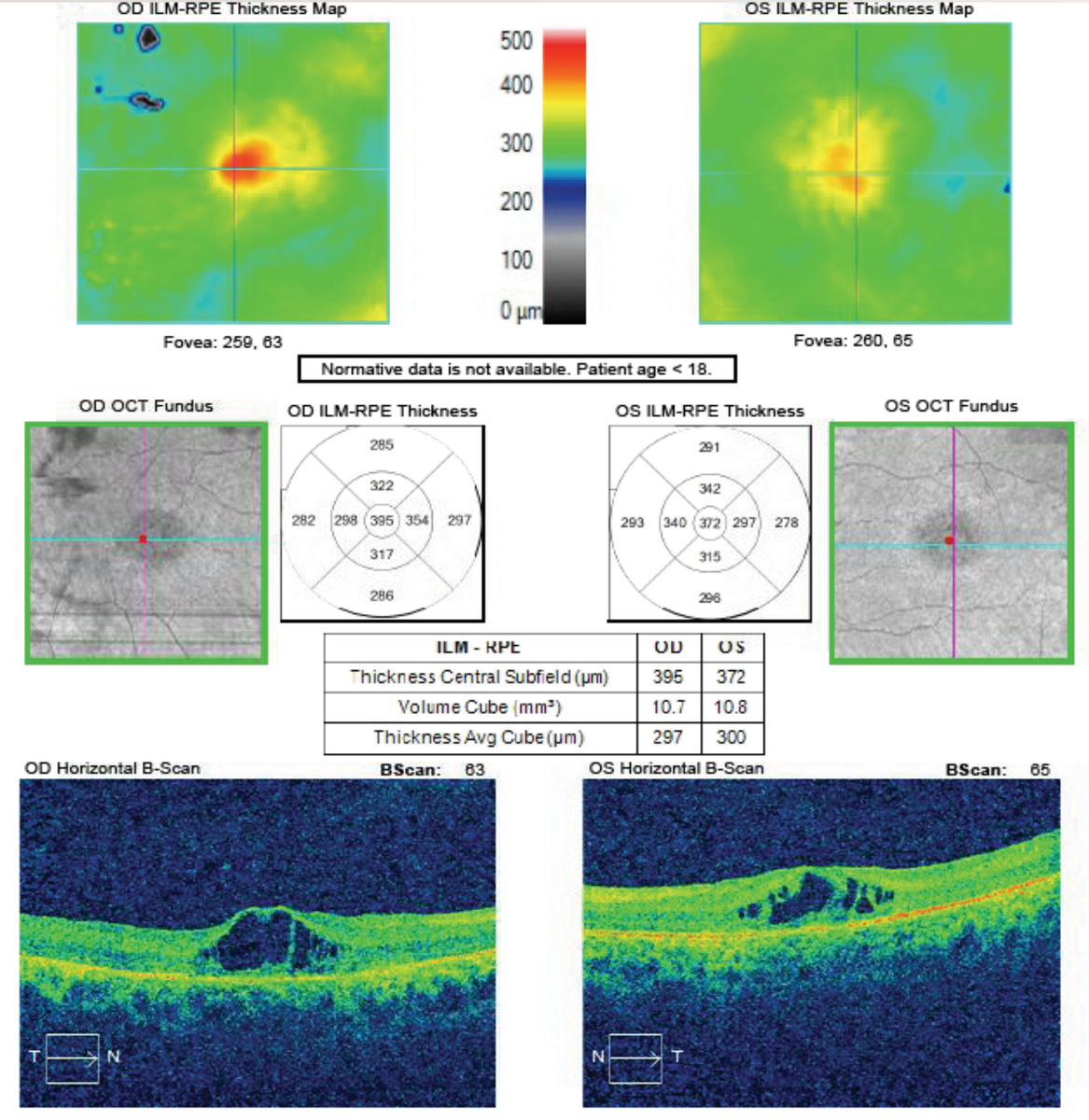
Case 1. Optic disc hypoplasia or refractive amblyopia? A six-year-old was referred for amblyopia. His BCVA was 20/20 in the right eye with a +3.00D and 20/70 in the left eye with +6.25-1.00x90 after cycloplegic refraction. There was no strabismus present on cover testing. As per standard of care, the patient was dilated. Retinal evaluation revealed asymmetric optic nerve head size with the left nerve being smaller when compared with the right (Figure 1). The patient was referred for imaging to rule out functional vs. pathological vision loss. OCT was performed but imaging from the left eye was unsuccessful (Figure 2). The patient returned a couple of weeks later, and the OCT image was repeated in the left eye only indicating a normal nerve with normal RNFL.
This case was in fact amblyopia with no retinal pathology and, although the optic nerve in the left eye appeared to be smaller, the appearance was due to higher hyperopia in the left eye and the OCT demonstrated that there was no pathological cause for the decrease in vision. This patient does have amblyopia secondary to anisometropia with a stronger prescription on the left eye.
 |
|
Fig 4. OCT of the macula OD and OS reveals CME in an 11-year-old boy referred for vision training due to reduced vision secondary to “learning disabilities.” Click image to enlarge. |
Case 2. Bilateral refractive amblyopia? A 13-year-old girl was referred for corneal topography secondary to high cylinder and the provider was “unable to refract past -6.00D cylinder, no lens attachment available, possible keratoconic component.”
BCVA was 20/30 in the right eye with +2.75-6.50x180. Visual acuity in the left eye was 20/25 with +3.25-6.00x015. Pinhole was done at this visit, and no improvement was noted. Slit lamp examination and dilation were unremarkable. Pentacam corneal topography demonstrated high astigmatism in both eyes but regular corneas (Figure 3a). The patient was diagnosed with refractive amblyopia in both eyes because of the high astigmatism.
The patient was referred for vision therapy three years later with complaints of eye strain and “history of refractive amblyopia.” BCVA was stable in the right eye at 20/30; however, the spectacle refraction had an increase of cylinder to +3.50-7.25x180. The left eye had a stable acuity of 20/30 and relatively stable refractive error of +2.50-6.75x005. A repeat of the Pentacam corneal topography was performed, and there was an increase in corneal cylinder of 1.4D in the right eye and 0.9D in the left eye, which is highly suspicious (Figure 3b). Gas permeable (GP) contact lenses were trialed, and the patient’s visual acuity improved. The patient was then subsequently fit with bitoric GP contact lenses with a final visual acuity that was 20/20- in the right eye and 20/25+ in the left eye.
While a formal diagnosis of keratoconus cannot be made in this case, the corneal topography is highly suspicious. BCVA cannot be assessed accurately unless you have attempted a GP lens. And unfortunately, this 13-year-old could have had better visual acuity in both eyes two years earlier if a GP lens was trialed. While these lenses may not be comfortable for young people, they deserve an attempt for the best possible visual acuity instead of being diagnosed with amblyopia. In this case, vision training is not necessary and would not have helped improve the vision.
 |
|
Fig. 5. Color fundus photos reveal attenuated arterioles (top), and the FAF photos reveal retinal hypo-AF indicating diffuse retinal degeneration and small hyper-AF rings in the macula, a sign of advanced retinitis pigmentosa (bottom). Click image to enlarge. |
In a separate unrelated case, a young woman in her 20s presented to an eye doctor who was temporarily covering for the regular eye doctor that day. The patient had complained of reduced vision in one eye for several weeks and was only correctable to 20/40 in the affected eye. The patient was a soft contact lens wearer. The eye doctor who was temporarily covering for the regular eye doctor concluded that the reduced vision was due to contact lens-induced corneal distortion. He advised the patient to discontinue her contact lens wear for one week and return when the regular eye doctor was there. The patient never returned, but as her vision continued to deteriorate in the affected eye, she went to several other specialists until she found an optometrist who performed a static visual field test, which revealed bitemporal hemianopic field loss due to what was ultimately determined to be a pituitary adenoma.
The patient sued all the eye doctors who had seen her prior to her visit with the optometrist who was the only one to perform the correct test, i.e., a static visual field test. And she also sued the eye doctor who saw her for only one visit as a temporary stand-in for the regular eye doctor. What could this eye doctor have done on that visit? Since the eye doctor suspected visual acuity loss secondary to “distortion from her contact lenses,” he could have placed a trial GP lens on her eye which would have become her “new cornea.” An improvement in the VA would have supported a corneal etiology for the reduced vision. In this case, however, the BCVA would not have improved because the patient had a space occupying visual pathway lesion that was the cause of the reduced vision. Therefore, using trial GP contact lenses can help weed out pathologies believed to be due to corneal issues.
Case 3. Bilateral refractive amblyopia with learning disability? An 11-year-old asymptomatic male was referred for a vision training work-up for possible refractive amblyopia and poor test taker with a history of learning difficulties. There was no family history of eye disease, and the patient reported no eye health history. The patient’s current prescription was OD +1.50-1.75x175 and OS +1.75-2.25x165. BCVA was 20/40 in each eye. The referring provider noted that the examination was unremarkable and that the macula was “clear.” The patient had not been dilated.
 |
|
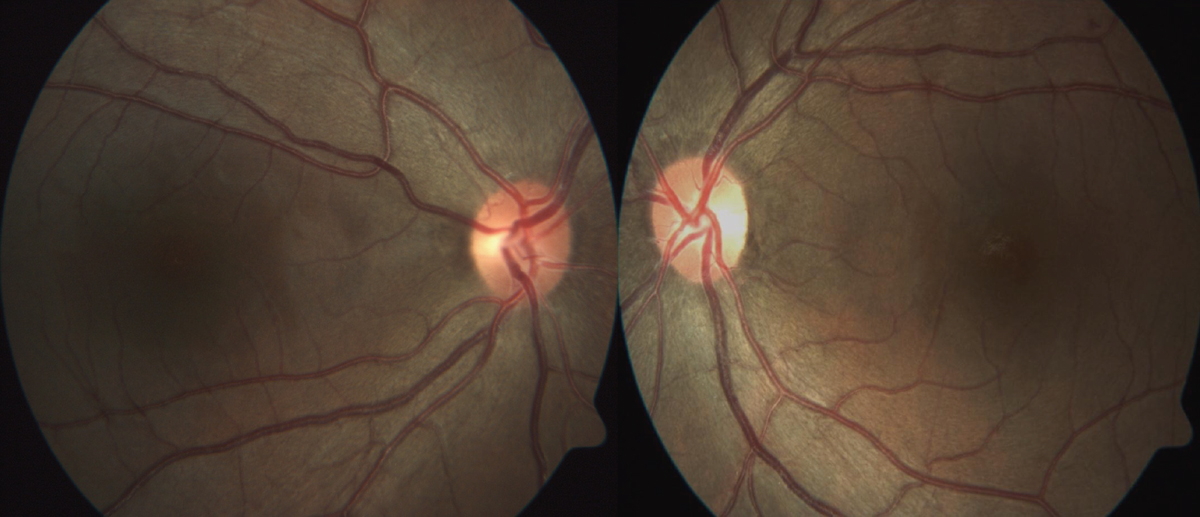
Fig. 6. Fundus photography reveals temporal pallor of both optic nerve heads, more obvious in the left eye. Click image to enlarge. |
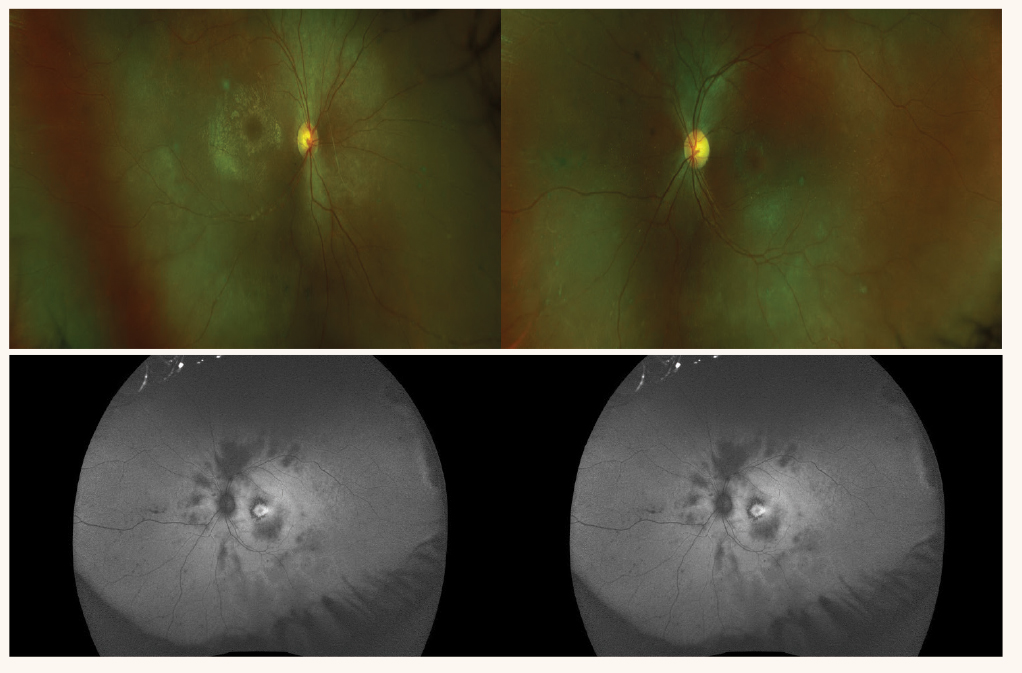
Examination through a dilated pupil revealed attenuated arterioles, lack of a foveal reflex but no pigment abnormalities (Figure 4). Fundus autofluorescence (FAF) imaging revealed areas of hypo autofluorescence (AF) in the posterior pole and a central, small hyper-AF ring in each eye (Figure 5). A macular OCT revealed a thickened macula in each eye with evidence of cystoid macular edema (CME). ERG testing revealed extinguished responses under both photopic and scotopic conditions. Genetic testing revealed two pathogenic variants in CRB1 which is associated with several autosomal recessive (ar) diseases including arRP.14 In this case, the genetic testing confirmed the clinical characteristics. CME is a late-stage development in arRP and the small hyper-AF ring seen in the FAF imaging confirms this is late-stage RP. Unfortunately, standard treatment of CME, including intravitreal injection of a steroid medication, failed to improve the CME.
Although the patient had a moderate degree of astigmatism, the numbers did not meet the criteria for refractive amblyopia. Therefore, the diagnosis of “possible refractive amblyopia” was not confirmed but we were able to correctly diagnose advanced RP. Not all patients with RP present with the typical bone-spicule pigmentation, especially in some pediatric cases.
All patients deserve a correct and timely diagnosis. Although there currently is no medical treatment, clinical trials are underway and during this patient’s lifetime, there may be treatment for him. Instead of vision training, this patient is better served by a low vision consult.
 |
|
Fig 7. 10º Octopus visual fields reveal a central scotoma in both eyes, greater in the left (red arrows). Click image to enlarge. |
Case 4. Possible bilateral amblyopia without pathology? A 31-year-old Hispanic female was referred for “possible bilateral amblyopia without pathology.” She reported decreased vision in both eyes for as long as she could remember. There was no family history of a similar issue. When she was younger, she had a full battery of tests, including an MRI to determine why she had reduced vision. None of these tests helped determine the cause. She was becoming concerned because now she has noted difficulty distinguishing colors on her computer screen. The referring doctor noted that her “ocular health was normal with a clear macula, and no pallor noted.” The doctor had performed a 30º visual field test which was noted to be “full OD and OS.”
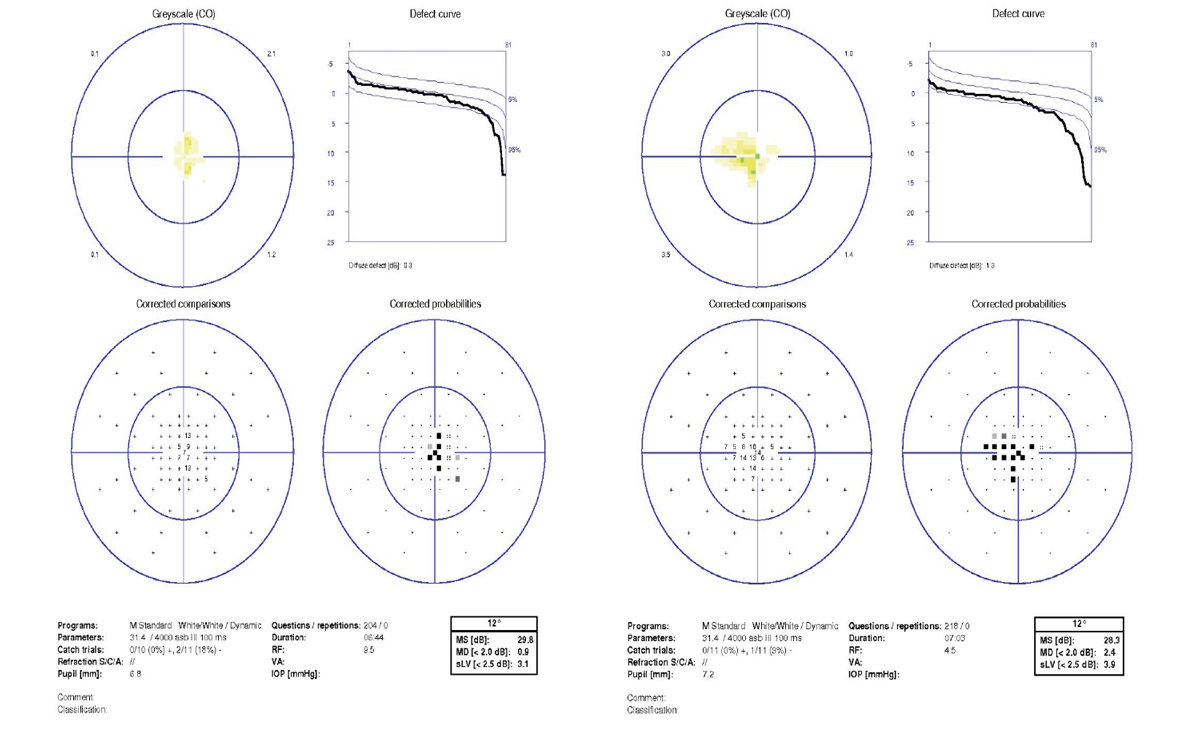
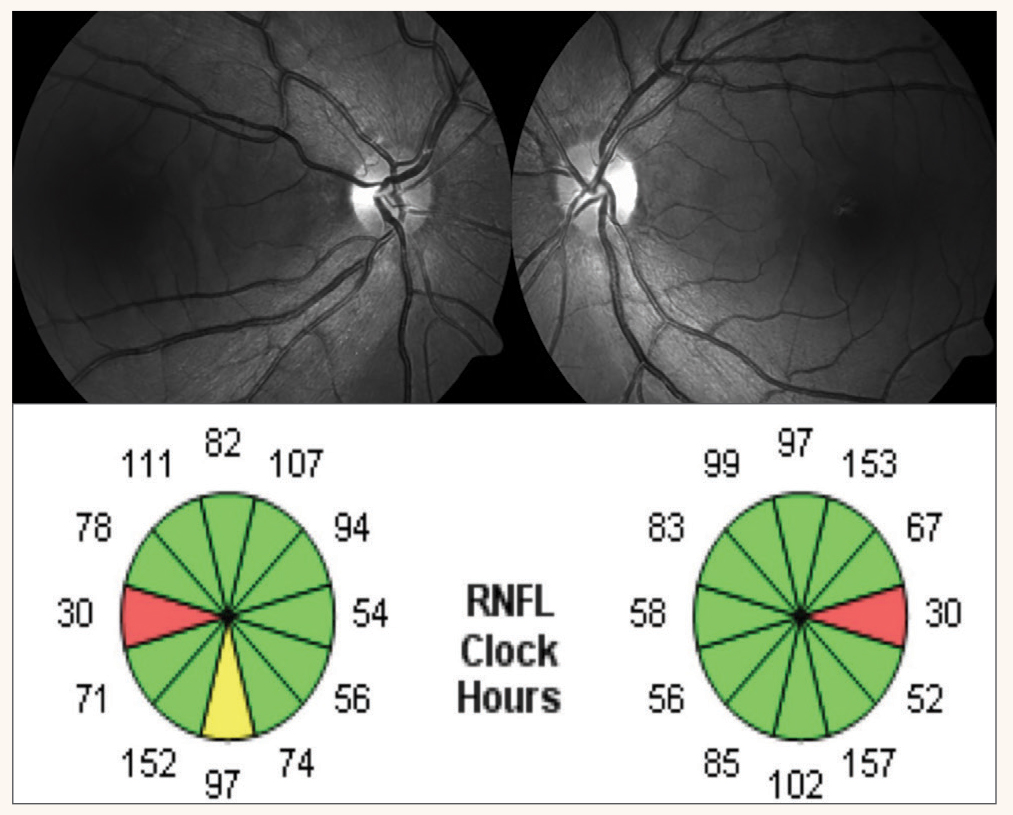
At her exam, BCVAs were 20/40 OD through a +0.50 sphere and 20/60 OS through plano -0.25x5. Pupils were normal, corneas and lenses were clear. Examination of the retinas revealed temporal pallor of both optic nerve heads, greater in the left eye (Figure 6). A 10º Octopus M TOP visual field test revealed small central scotomas in both eyes, greater in the left eye (Figure 7). An OCT of the RNFL revealed thinning along the clock sectors representing the papillomacular bundle RNFL in both eyes (Figure 8). Due to the suspicion of a hereditary optic neuropathy, genetic testing was performed, which revealed a pathogenic variant in the OPA1 gene, which is associated with dominant optic atrophy or Kjer’s disease.15
The visual field performed in the referring doctor’s office was a 30° visual field. Why did it miss a central field defect? The points in a 30° field test are six degrees apart and a small central scotoma would easily be missed on a test program like this one. The Octopus M TOP visual field test is a 10º field test in which the points tested within the central 4° of the visual field are 0.7° apart. That is why this test program was able to detect small central scotomas that explained the patient’s decreased visual acuity. BCVA was worse in the left eye (20/60) than the right eye (20/40). The Octopus M TOP visual field test confirmed that the central scotoma was greater in the left eye.
 |
|
Fig. 8. OCT RNFL clock sector reveals symmetric papillomacular bundle thinning coinciding with the temporal disc pallor and central visual field defects in each eye. Click image to enlarge. |
Differentiating functional from pathological vision loss can best be determined when ordering the proper tests. The referring practitioner did not order an OCT of the RNFL which would have detected thinning seen in Figure 8. In addition, the practitioner ordered a 30° visual field test but when it was found to be “full,” the practitioner should have had the patient return for a 10° visual field test. When a patient has unexplainable loss of vision in one eye, it is best to initially perform a 10° field test.
Takeaways
The cases presented in this review are but a few of the many where vision loss has been attributed to functional issues. The missed pathology can result in a delay in diagnosis which can result in vision loss that can be treatable. In other cases, the true nature of the disease is not identified, and this can affect services that could be made available to the patient, such as low vision.
In the case of unidentified inherited retinal disease, the reason for the vision loss was not accurately diagnosed, and with the development and approval of gene therapies, this could have consequences in the future. In addition, families need to be aware of the history of eye diseases for purposes of family planning. Of greatest consequence is the misdiagnosis of pathology that can result in permanent vision loss or even life because the condition was not treated in time, as in the case of visual pathway lesions that grow if undetected and untreated.
Eyecare practitioners need to rule out disease before using the catchall diagnosis of amblyopia. While a thorough eye examination is the first place to start, knowledge of amblyogenic factors and the use of current imaging technologies are extremely helpful to avoid these types of errors. In addition, the proper use of static perimetry in place of gross CVFs is essential to help detect early visual pathway disease in unexplainable acuity loss, especially in one eye in an individual over the age of eight years and where no amblyogenic factor is present. Despite this, gross CVF testing is still taught in schools today and is still used throughout the eyecare profession.
Dr. Bass is a Distinguished Teaching Professor at the SUNY College of Optometry. She is an attending in the Retina and Electrodiagnostic Clinics in the University Eye Center. She has no financial disclosures.
Dr. Rutner is an Associate Clinical Professor at SUNY College of Optometry. She is Chief of Vision Rehabilitation, teaches and does clinical research. She has received grant support from the Hoffman Foundation Grant and is a speaker for Apply EBP.
1. Optometric clinical practice guideline: care of patients with amblyopia. American Optometric Association. www.aoa.org/aoa/documents/practice%20management/clinical%20guidelines/consensus-based%20guidelines/care%20of%20patient%20with%20amblyopia.pdf. Accessed June 4, 2024. 2. Bonneh YS, Sagi D, Polat U. Spatial and temporal crowding in amblyopia. Vision Res. 2007;47(14):1950-62. 3. Ghasia F, Wang J. Amblyopia and fixation eye movements. J Neurol Sci. 2022;441:120373. 4. Jia Y, Ye Q, Zhang S, et al. Contrast sensitivity and stereoacuity in successfully treated refractive amblyopia. Invest Ophthalmol Vis Sci. 2022;63(1):6. 5. Chen AM, Manh V, Candy TR. Longitudinal evaluation of accommodation during treatment for unilateral amblyopia. Invest Ophthalmol Vis Sci. 2018;59(5):2187-96. 6. Tarczy-Hornoch K, Varma R, Cotter SA, et al. Risk factors for decreased visual acuity in preschool children: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118(11):2262-73. 7. Marinoff R, Vricella M, Duckman RH. Visual acuity in the young child. In: Schnell P, Taub MB, Duckman RH, eds. Visual development, diagnosis and treatment of the pediatric patient. Lippincott Williams & Wilkins, 2019. 8. Rutner D, Bartolone A. Quantification of refractive error. In: Schnell P, Taub MB, Duckman RH, eds. Visual development, diagnosis and treatment of the pediatric patient. Lippincott Williams & Wilkins, 2019. 9. Yazdani N, Sadeghi R, Momeni-Moghaddam H, et al. Comparison of cyclopentolate vs. tropicamide cycloplegia: a systematic review and meta-analysis. J Optom. 2018;11(3):135-43. 10. Jin J, Apple A, Friess A, et al. Using OCT fixation shift to assess eccentric fixation in children with residual amblyopia. Transl Vis Sci Technol. 2020;9(12):30. 11. Wright KW. Sensory aspects of strabismus. In: Wright KW, Spiegel PH, eds. Pediatric ophthalmology and strabismus. Springer Science & Business Media, 2013. 12. Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: basic and clinical aspects. Butterworth-Heinemann, 1991. 13. Bass SJ, Rutner D. The differential of pathological from functional vision loss. Adv Ophthalmol Optom. 2023;8(1):27-44. 14. Bujakowska K, Audo I, Mohand-Saïd S, et al. CRB1 mutations in inherited retinal dystrophies. Hum Mutat. 2012;33(2):306-15. 15. Lenaers G, Hamel C, Delettre C, et al. Dominant optic atrophy. Orphanet J Rare Dis. 2012;7:46. |
