What Specular Microscopy Reveals About Your Patients
Visualizing the corneal endothelium aids in the diagnosis and management of myriad diseases.
By Daniel Epshtein, OD
 |
Release Date: September 15, 2019
Expiration Date: September 15, 2022
Estimated time to complete activity: 1 hour
Jointly provided by Postgraduate Institute for Medicine and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
- Evaluate the corneal endothelium, and be able to differentiate between normal endothelial structure vs. abnormal endothelial structure.
- Identify the symptoms of moderate to advanced endothelial disease, such as blurred vision, fluctuating vision, or permanent visual impairment.
- Explain the importance of endothelial cell density, and the reasons why it may change.
- Recognize the characteristics of different corneal endotheliopathies using specular microscopy, including corneal guttata, Fuch’s endothelial dystrophy, age-related endotheliopathy, contact lens-induced endotheliopathy, and others.
Target Audience: This activity is intended for optometrists engaged in the care of patients with a compromised corneal endothelium.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Daniel Epshtein, OD, Mount Sinai St. Luke’s, New York, NY.
Credit Statement: This course is COPE approved for 1 hour of CE credit. Course ID is 64431-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Epshtein: Has received fees for non-CME/CE services from Carl Zeiss Meditec.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
The corneal endothelium is responsible for maintaining the delicate balance of stromal hydration to ensure adequate nutrition without sacrificing corneal clarity. The endothelium can be visualized by using specular reflection at the slit lamp. Though this technique is essential to the evaluation of the cornea, the slit lamp’s limited magnification often makes it difficult to assess subtle endothelial changes.
Specular microscopy is a noninvasive imaging technique that produces high-magnification images of the corneal endothelium. These images can be analyzed qualitatively and quantitatively (using automated software) to help diagnose pathology, accurately monitor endothelial disease and aid in surgical comanagement.
 |
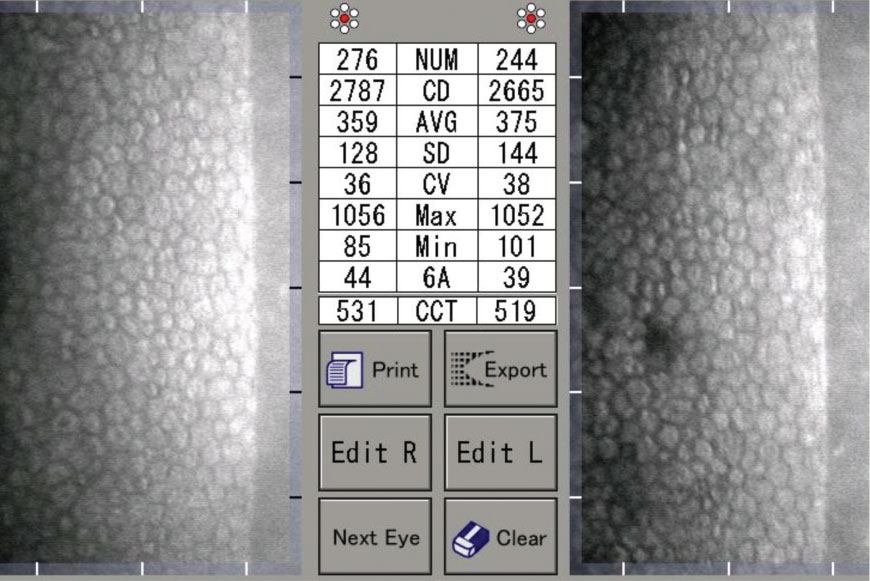
| A specular microscopy report of a normal 30-year-old male. Note the uniform hexagonal cells, high CD, low CV and high HEX/6A. Click image to enlarge. |
Corneal Endothelium
The corneal endothelium is a nonregenerative (though some evidence suggests that in vivo regeneration may be possible) single layer of cells comprising the most posterior portion of the cornea.1 In a young and healthy individual, this monolayer is a relatively uniform mosaic of 500,000 hexagonally-shaped cells.2
The primary physiological purpose of the corneal endothelium is to create a porous barrier between the corneal stroma and aqueous humor. Due to the cornea’s avascularity, its nutrition is derived from passive diffusion of the tear film and aqueous humor into the cornea.2 To maintain proper corneal function, a relatively low amount of hydration is necessary, though the stroma tends to overhydrate under normal physiological conditions.
Overhydration of the stroma, clinically known as corneal edema, reduces corneal transparency and visual function. The passive flow of aqueous humor into the cornea is offset by active transportation via pumps in the corneal endothelium.
As endothelial cells age and slough off, neighboring cells stretch, relocate or fuse to maintain the leaky barrier. This process results in pleomorphism (cell shape variability) and polymegethism (cell size variability). Though both processes correlate with decreased corneal endothelial function, there is no direct relationship between the amounts of pleomorphism and polymegethism, suggesting that there is a variable structure-function relationship.3 The normal deterioration of the corneal endothelium can be accelerated by primary corneal endotheliopathies such as Fuchs’ corneal dystrophy or secondary corneal endotheliopathies such as traumatic, surgical, contact lens-induced and inflammatory.
Table 1. Expected Cell Density Based on Decade of Life | |
| Age | Average CS (cells/mm2) |
| 10-19 | 2,900-3,500 |
| 20-29 | 2,600-3,400 |
| 30-39 | 2,400-2,300 |
| 40-49 | 2,300-3,100 |
| 50-59 | 2,100-2,900 |
| 60-69 | 2,000-2,800 |
| 70-79 | 1,800-2,600 |
| 80-89 | 1,500-2,300 |
Specular Microscopy
This diagnostic tool visualizes the corneal endothelium by taking advantage of specular reflection at the interface between the endothelium and the aqueous humor. This is accomplished with white light, similar to that used in slit lamp biomicroscopy, and a high magnification microscope. Only light at a specific plane (the plane of the endothelium) is captured, so any irregularities in the smooth planar surface of the endothelium are seen as hyper-reflective (more reflective) or hypo-reflective (less reflective) areas.
Hyper-reflective areas can signify inflammatory cells while hypo-reflective areas (which are more common) can denote guttata, endothelial pigment or endothelial swelling. Most specular microscopes also measure the central corneal thickness, which can serve as a biomarker of corneal endothelial function.
After imaging, an automated algorithm segments and counts the endothelial cells. From this, the specular microscope generates various indices that aid in the diagnosis and management of corneal disease. The cell density (CD), coefficient of variation (CV), variability in hexagonal shape (HEX) and central corneal thickness (CCT) are the most important indices when evaluating for endotheliopathies.
Corneal Endotheliopathies
The quantitative and qualitative analysis of specular microscopy images can help in the diagnosis and management of various corneal endotheliopathies:
Age-related endothelial degeneration. As the cornea ages, the normal loss of endothelial cells with resultant pleomorphism and polymegethism can be detected. Guttata are often present and usually increase in number and size with time. Without disease or injury, age-related degeneration will not lead to corneal decompensation. The CD will decrease at a rate of 0.6% per year but never below the critical density of 300 to 600cells/mm2 (Table 1). The CV will increase and the HEX/6A will decrease as well, but no appreciable corneal edema should be detected clinically or with serial CCT. Due to commonly comorbid cataracts, age-related corneal endothelial degeneration should be assessed to determine the risk of corneal decompensation with cataract surgery.
Fuchs’ endothelial corneal dystrophy. This is a non-inflammatory bilateral corneal endothelial dystrophy characterized by progressive corneal endothelial degeneration and guttata. This condition is autosomal dominant but can be due to a sporadic mutation as well; the genetics are not well identified.2 The guttata in Fuchs’ endothelial corneal dystrophy tend to be central, with peripheral guttata appearing later in the disease process.
Early in the disease, patients may complain of poor vision or glare in the morning, which resolves by the early afternoon. This variation in vision is due to the decreased tear film evaporation with prolonged eyelid closure (during sleep), which increases hydration of the cornea. This physiological corneal edema, which occurs in all individuals, cannot be compensated for by the reduced function of the endothelium. As Fuchs’ dystrophy progresses, endothelial function decreases and the corneal edema worsens.
| Specular Microscopy Indices Defined Below is a quick summary on the various quantitative indices generated with specular microscopy. Cell density (CD): The number of endothelial cells per mm2. Cell density decreases with age and disease. When evaluating the cell density, it is important to consider age-expected values, as anything below the age-expected average value may be an indicator of underlying disease. Patients with a cell density less than 1,000 cells/mm2 are at higher risk for developing pseudophakic bullous keratopathy.4 To maintain proper hydration of the cornea, 300 to 600 cells/mm2 are necessary.2 When cell density falls below this critical number, the balance of stromal hydration is tilted toward corneal edema and loss of corneal transparency. Coefficient of variation (CV): Represents the amount of variation in cell size. The coefficient of variation is a measure of polymegethism, which occurs during corneal endothelial repair. A coefficient of variation less than 33 is considered normal. An elevated coefficient of variation is often considered an early sign of endothelial disease, as this is a marker of endothelial cell remodeling. HEX or 6A: Represents the number of cells that have a hexagonal shape. Pleomorphism is the decrease of hexagonal cells within the corneal endothelium. As pleomorphism increases, the barrier function of the corneal endothelium decreases. A HEX or 6A less than 50% is considered abnormal. Central corneal thickness (CCT): The central corneal thickness measured during image acquisition. A thickened central cornea may denote corneal edema and reduced endothelial function. Number of cells counted (NUM): The number of cells counted in the analysis. Visualization of imaging of endothelial cells in diseased corneas may be difficult, so it is important that an adequate number of cells are included in the quantitative analysis. Average cell area (AVG): Measurement of the average cell area. This number increases with age as polymegathism increases. Standard deviation of mean cell area (SD): The standard deviation of mean cell area within the analysis. |
Decreasing CD and HEX/6A and increasing CV will be noted before corneal edema is detected clinically or with serial CCT. As the CD and HEX/6A continue to decrease, and the CV and the number of guttata increase, the cornea decompensates and corneal edema is noted. This leads to significant fluctuation in vision and reduced vision. Even without the presence of corneal edema, guttata can lead to glare and subjectively reduced vision.5,6 It is important to monitor the CCT during serial imaging to detect corneal edema. Though the CD, CV, HEX/6A and appearance of the corneal endothelium are related to the risk of corneal edema, a perfect structure-function relationship does not exist. Corneal edema can occur with seemingly moderate specular microscopy changes and may not occur with advances changes.
Posterior polymorphous dystrophy. This non-inflammatory bilateral corneal endothelial and Descemet’s membrane dystrophy is characterized by epithelial-like cells within the endothelium. This condition is autosomal dominant and is usually diagnosed during early to mid-childhood due to the presence of corneal opacities, edema and blurred vision. The pathology noted on presentation is usually nonprogressive or slowly progressive.
Clinically, these patients will have various blue-gray lesions of the endothelium that can be round, confluent, isolated or band-like. Specular microscopy will reveal irregular cells with scalloped edges that do not resemble the normal uniform hexagonal mosaic of endothelial cells.
Iatrogenic corneal endotheliopathy. Damage to the endothelium can occur with various intraocular surgeries. During routine cataract surgery, phacoemulsification energy directly damages the corneal endothelium. Intraoperative contact of the endothelium with various instruments or intracameral fluids can also lead to endothelial cell loss. Uncomplicated cataract surgery often leads to a 10% decrease in CD.7
Significant intraocular pressure spikes intraoperatively or perioperatively have been theorized as other possible etiologies of endothelial damage. Glaucoma drainage devices such as tubes or micro-stents have also been implicated in postoperative iatrogenic corneal endotheliopathy. Especially in patients with micro-stents, be sure to repeat specular microscopy periodically to evaluate corneal endothelial integrity.8
 |
| Moderate (left) and advanced (right) Fuchs’ corneal endothelial dystrophy. Note the asymmetric CCT of the patient on the right. Click image to enlarge. |
Preoperatively, it is important to clinically scrutinize the corneal endothelium to ensure there are no risk factors for postoperative corneal decompensation. Many surgeons and comanaging optometrists include specular microscopy in their presurgical evaluation. Preoperatively, the presence of a low CD, low HEX/6A, numerous or confluent guttata or high CV is considered a risk factor for pseudophakic bullous keratopathy. In patients without endothelial disease, the rate of pseudophakic bullous keratopathy is 1% to 2%, but the rate increases to 14% in patients with a CD less than 1,000cells/mm2.9
Patients who develop pseudophakic bullous keratopathy usually complain of glare and reduced vision, and they will have clinically appreciable corneal edema. The corneal edema in pseudophakic bullous keratopathy starts in the stroma but eventually leads to the formation of fluid-filled bullae within the epithelium and subepithelial space. These bullae are in direct contact with corneal nerves leading to sensations of pain, irritation and foreign body. The bullae can then burst, leading to epithelial breaks, which are extremely painful due to the exposure of corneal nerve endings. Cases that cannot be treated medically will have permanent vision loss and/or require penetrating or endothelial keratoplasty.
Postoperatively, pseudoguttata may be noted due to corneal edema and inflammation. Pseudoguttata changes mimic guttata as seen in Fuchs’ corneal endothelial dystrophy but are transient and related to anterior segment inflammation. Pseudoguttata resolve as the inflammation lessens. The hypo-reflective areas of pseudoguttata are not caused by actual structures extending from the cornea (such as guttata) but are due to cellular edema, which alters the planar surface of the endothelium, reducing reflection.
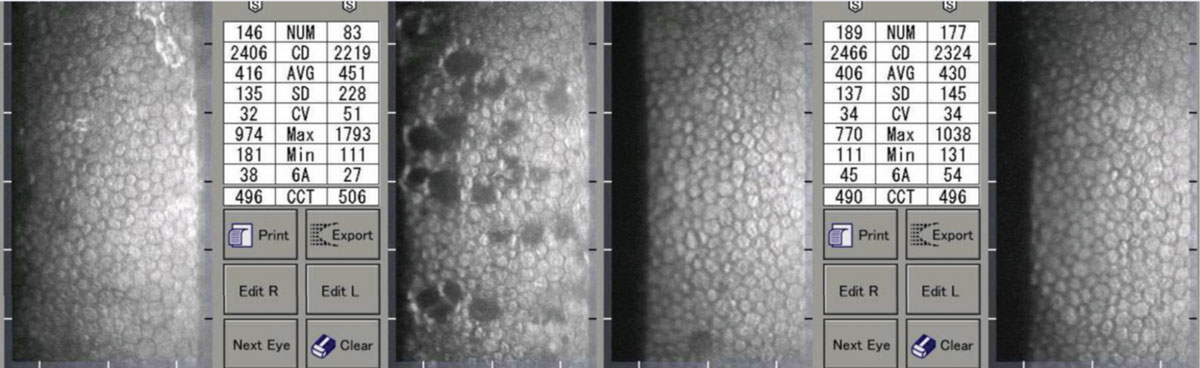 |
| A patient with unilateral iritis OS. Note the resolution of the pseudoguttata and improvement in indices. Click image to enlarge. |
Inflammation. As with postoperative corneal endothelial changes, anterior segment inflammation such as uveitis or corneal endotheliitis can lead to pseudoguttata formation. These changes usually correlate with inflammation severity. Chronically, anterior segment inflammation can damage the corneal endothelium via immune pathway proteins or direct infiltration of inflammatory cells into the endothelium. Repeat episodes of anterior segment inflammation can lead to endothelial degeneration, reduced CD and HEX/6A, increased CV and an increased amount of guttata.
It is important to perform specular microscopy on patients with repeat inflammation because treatment regimens may need to be altered if there is significant corneal endotheliopathy.
Trauma. Nonpenetrating trauma of the cornea can lead to corneal endothelial edema that resolves after several days. Acutely, the edema can be noted with biomicroscopy, but after resolution, corneal endothelial damage can usually only be detected with specular microscopy. A decreased CD is most often noted in areas of damage.
Glaucoma. Patients with glaucoma have been noted to have a lower CD and quicker rate of CD loss than age-matched norms.10,11 It’s unclear whether the increased intraocular pressure directly causes these changes or if a metabolic insult, such as hypoxia caused by the increased intraocular pressure, is the culprit. Toxicity to topical antihypertensives has also been considered as a possible etiology.
Post-penetrating keratoplasty/endothelial keratoplasty. Specular microscopy is essential in determining whether donor corneal tissue is adequate for transplantation and for annual (or more often) evaluation of graft endothelial structure and function. There is a dual-phase rapid loss of CD after corneal transplantation; a 7.8% per year CD decrease during the first three to five years and a 4.2% per year CD decrease in years six to 10.2 As with Fuchs’ corneal dystrophy, CD and HEX/6A decrease while CV and guttata presence increases.
 |
| A 40-year-old patient with a history of low Dk contact lens use. Click image to enlarge. |
Contact Lens-Induced Endotheliopathy
Acutely, the insertion of contact lenses (especially scleral lenses) can create pseudoguttata or endothelial blebs. These blebs often disappear after minutes to hours, or with corneal adaptation to contact lens wear. These changes are always reversible with contact lens removal. Because similar changes occur during prolonged lid closure, researchers hypothesize that this is a hypoxic event.2 The corneal endothelium swells, leading to changes similar to those in anterior segment inflammation.
Long-term contact lens wear leads to increased pleomorphism and polymegethism, which correlates with decreased HEX/6A, increased CV and the presence of guttata. Chronic contact lens–induced endotheliopathy is much more common in low Dk lenses. The amount of corneal degeneration correlates with the length of wear.
Due to the corneal endothelium’s nonregenerative nature, contact lens-induced endotheliopathy persists even after contact lens wear is discontinued or a higher Dk material is prescribed. Mild improvement in HEX/6A and CV may be noted after discontinuation of low Dk lenses, but this is due to a decrease in edema and improved visualization of the corneal endothelial mosaic. It is important to check specular microscopy in patients with a history of low Dk lens wear, blurred vision with contact lens wear, fluctuating vision or corneal edema.
A healthy corneal endothelium is imperative to corneal clarity and clear vision. Though visualization of the corneal endothelium is often inadequate with slit lamp biomicroscopy, specular microscopy is a useful, noninvasive modality that can be easily incorporated into any optometric practice. The precise visualization of the corneal endothelium and the use of quantitative analyses aids in diagnosis and management of myriad corneal diseases in primary care practices, disease-oriented practices and those active in comanagement.
Dr. Epshtein practices in the ophthalmology department of Mount Sinai St. Luke’s in New York. Previously, he held a position in a high-volume multispecialty ophthalmology practice where he supervised fourth-year optometry students as an adjunct assistant clinical professor of the SUNY College of Optometry. His research focuses on using the latest ophthalmic imaging technologies to elucidate ocular disease processes and to simplify equivocal clinical diagnoses.
|
1. Van den Bogerd B, Dhubhghaill SN, Koppen C, et al. A review of the evidence for in vivo corneal endothelial regeneration. Surv Ophthalmol. 2018;63(2):149-165. 2. Copeland RA, Afshari NA, Dohlman, CH. Copeland and Afshari’s Principles and Practice of Cornea. Vol. 1. New Delhi: Jaypee Brothers Medical Publishers; 2013. 3. Rao GN, Shaw EL, Arthur EJ, Aquavella JV. Endothelial cell morphology and corneal deturgescence. Ann Ophthalmol. 1979;11(6):885-99. 4. Corneal endothelial photography. Three-year revision. American Academy of Ophthalmology. Ophthalmology. 1997;104(8):1360-5. 5. Watanabe S, Oie Y, Fujimoto H, et al. Relationship between corneal guttae and quality of vision in patients with mild Fuchs’ endothelial corneal dystrophy. Ophthalmology. 2015;122(10):2103-9. 6. Oie Y, Watanabe S, Nishida K. Evaluation of visual quality in patients with Fuchs endothelial corneal dystrophy. Cornea. 2016;35(Suppl 1):S55-S58. 7. Bourne RR, Minassian DC, Dart JK, et al. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004;111(4):679-85. 8. FDA Safety Communication. Update: Potential eye damage from Alcon CyPass micro-stent used to treat open-angle glaucoma. www.fda.gov/medical-devices/safety-communications/update-potential-eye-damage-alcon-cypass-micro-stent-used-treat-open-angle-glaucoma-fda-safety. October 24, 2018. Accessed August 29, 2019. 9. Yamazoe K, Yamaguchi T, Hotta K, et al. Outcomes of cataract surgery in eyes with a low corneal endothelial cell density. J Cataract Refract Surg. 2011;37(12):2130-6. 10. Sihota R, Lakshmaiah NC, Titiyal JS, et al. Corneal endothelial status in the subtypes of primary angle closure glaucoma. Clin Exp Ophthalmol. 2003;31(6):492-5. 11. Cho SW, Kim JM, Choi CY, Park KH. Changes in corneal endothelial cell density in patients with normal-tension glaucoma. Jpn J Ophthalmol. 2009;53(6):569-573. |
