 |
What ODs Need to Know About Thyroid Eye Disease
Understanding how to approach the diagnosis and management of this condition is a key role of the primary eyecare provider.
By Michael Carstens, OD
Release Date: October 15, 2022
Expiration Date: October 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
Identify thyroid eye disease among their patients.
Determine when and how they should use clinical activity scores.
Determine the best treatment approach for their patients.
Recognize when to get additional labs and/or imaging, or when to refer.
Target Audience: This activity is intended for optometrists engaged in thyroid eye disease management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Michael Carstens, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #124729 and course ID 80828-SD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Author: Dr. Carstens has no financial interests to disclose. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
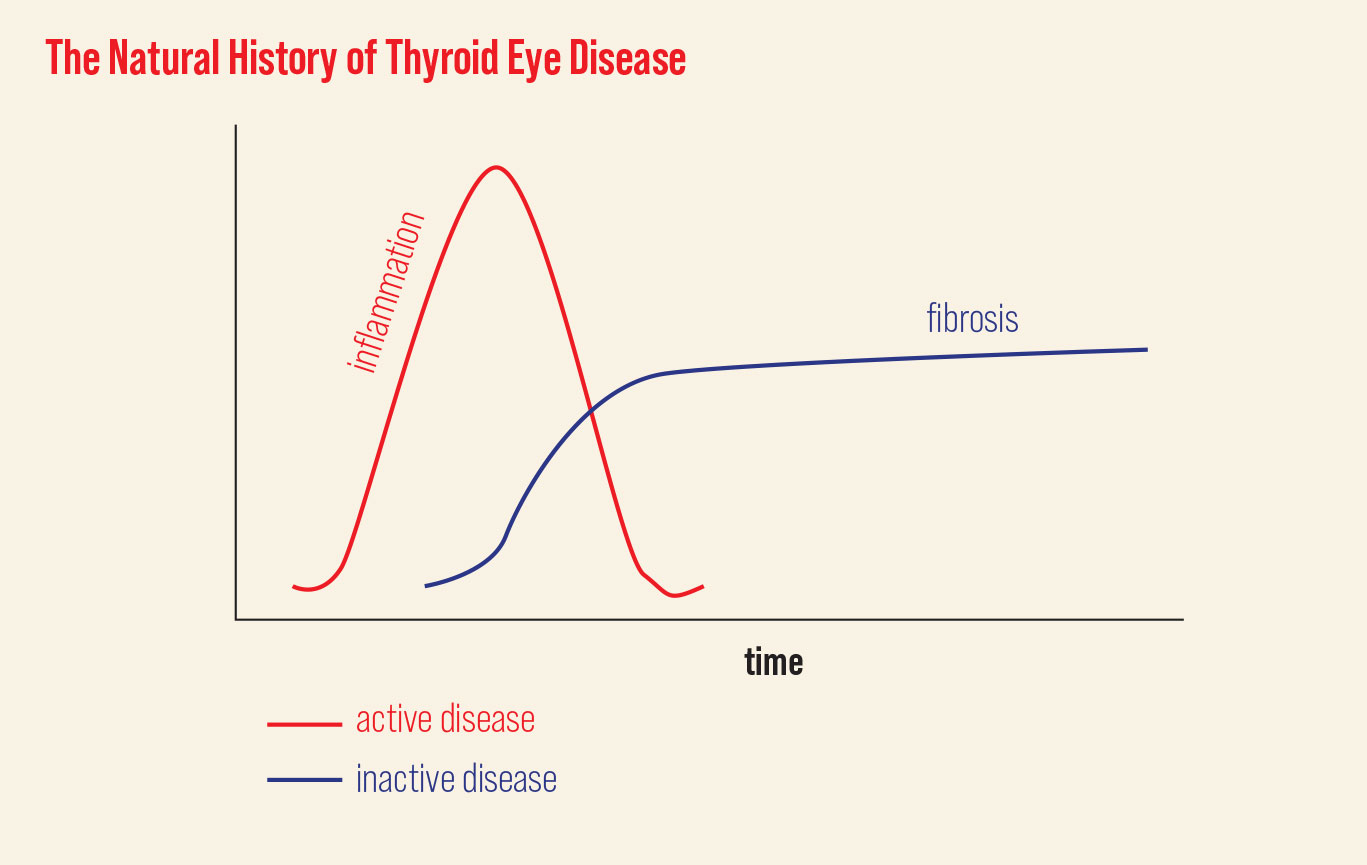 |
| TED has two different phases: an active and an inactive. Click image to enlarge. |
Thyroid eye disease (TED) has been called many names such as thyroid-associated orbitopathy, dysthyroid orbitopathy and thyrotoxic exophthalmos. TED is commonly associated with Graves’ disease (GD), which is used synonymously with Graves’ orbitopathy and Graves’ ophthalmopathy in the literature.
GD or Graves’ hyperthyroidism is a relatively common autoimmune disease affecting somewhere between 1% and 3% of the adult population. The onset of GD is typically between the ages of 30 and 50. It’s more common in women than men; however, males and those over 50 tend to develop more severe disease.1 Around 20% to 50% of GD patients will develop ophthalmologic signs, usually within two years of diagnosis of thyroid disease. Approximately 90% of TED patients will have hyperthyroidism, and the remainder will be euthyroid or have TED associated with Hashimoto’s disease or secondary to primary hyperthyroidism.1 Around 40% will present clinical signs before or at the time of diagnosis.1,2
As an optometrist, you are likely to encounter several patients with TED over the course of your career. Familiarizing yourself with the clinical signs and symptoms and diagnostic testing as well as the latest management strategies can significantly improve your patients’ outcomes and quality of life.
Pathogenesis of TED
Erroneous and excessive orbital fibroblast (OF) activity has been identified as the primary mechanism in Grave’s orbitopathy. Fibroblasts, derived from mesenchymal stem cells in the bone marrow, can reside in connective tissue or can be found circulating throughout the body. They are most commonly associated with structural maintenance and produce extracellular matrix proteins such as collagen and glycosaminoglycans. However, OF also play a significant role in the immune response to tissue injury. They are capable of activating T-cells through antigen presentation and cytokine secretion; in turn, T-cells engage in regulating OF activity and differentiation.
The link between the thyroid and OF is not well understood. However, distinct populations of orbital fibroblasts of GD patients have been identified, and these cells vary in their capacities for gene expression, cytokine release and differentiation.3 Orbital fibroblasts, especially those from GD patients, can be hyper-responsive when compared with fibroblasts from other anatomical locations.4
It has recently been shown that orbital fibroblasts, especially those of GD patients, display both thyrotropin (TSH) and insulin-like growth factor-1 receptors (IGF-1). These receptors reside in close proximity of one another on the cell surface, and physical scaffolds between them enable cross-talk.5 Stimulation of this TSH-IGF-1 receptor complex results in the secretion of hyaluronic acid and cytokines. Thus, the IGF-1 receptor has also been implicated in the pathogenesis of TED. Teprotumumab exploits the IGF-1 receptor pathway in its mechanism of action and will be discussed later.
During the inflammatory phase of GD, excessive production of extracellular matrix proteins, notably the highly hydrophilic HA, from activated fibroblasts leads to connective tissue and extraocular muscle edema and dysfunction. Muscle swelling along with an increase in adipogenesis (by way of fibroblast differentiation) results in an overall increase in orbital tissue volume. Due to the rigid confines of the bony orbital walls, the increase in volume pushes the globe forward and creates congestion at the orbital apex.
As the acute inflammatory phase resolves, tissue remodeling and fibrosis proceeds while the orbital fibroblasts continue to differentiate into adipocytes and myofibrocytes. The result can be permanent motility dysfunction and disfigurement and should be avoided at all costs.
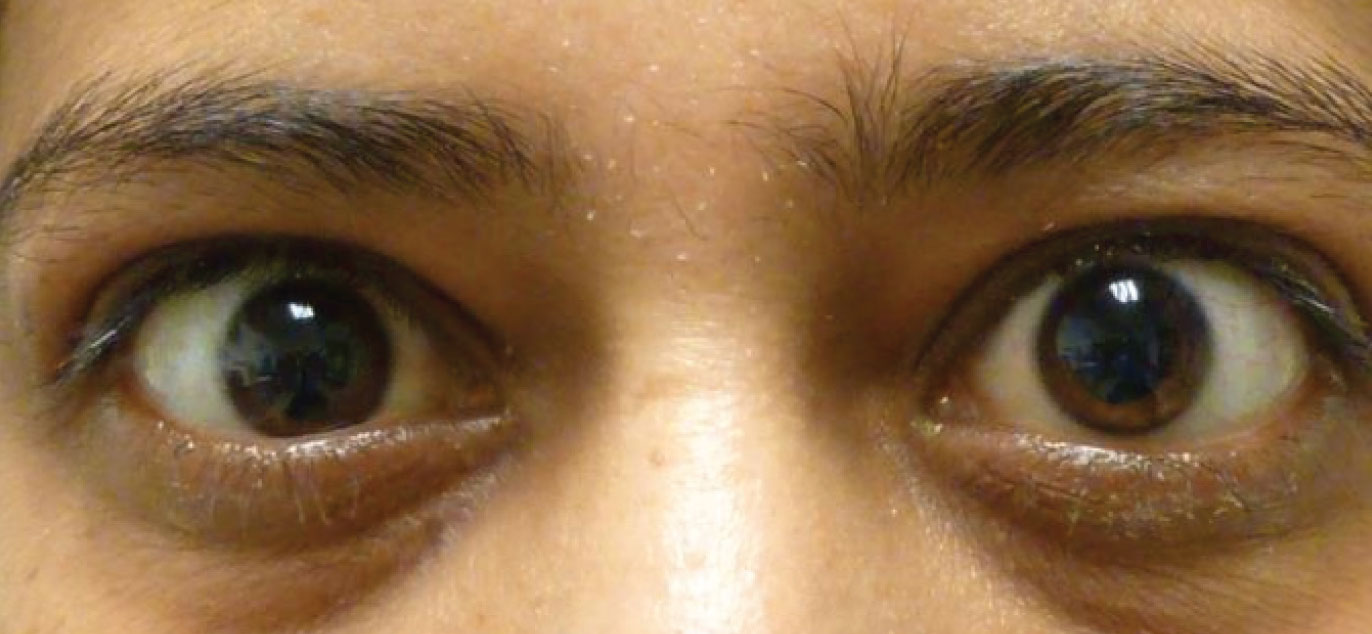 |
| Mild lid retraction with temporal flare. Photo: Michael Richard, MD. Click image to enlarge. |
Natural History
This can be categorized by two phases: the initial inflammatory phase, or active phase, followed by an inactive or fibrotic phase. During the active phase, clinical signs and symptoms can progress rather rapidly before quieting spontaneously after six to 18 months. Although inflammation subsides, patients rarely return to their baseline in the inactive state. It’s important to understand the different management strategies for both phases of the disease, as treatment and monitoring vary greatly.
Two subtypes of TED have been described, and their clinical courses can differ substantially. Type I tends to be found in younger patients with a whiter and quieter presentation, less muscle involvement and more fat deposition in the orbit. As a result, these patients are less likely to develop diplopia and dysthyroid optic neuropathy (DON). Type II is more likely to occur in older patients with more overt anterior segment inflammation such as conjunctival injection, eyelid edema and chemosis. These patients tend to have more muscle involvement and are at higher risk of DON. Smokers are more likely to fall into the type II category.
TED in the Clinic
Evaluation of a patient with suspected TED should start with a detailed history including a review of systems with specific questions relating to thyroid disfunction. For hyperthyroidism, ask about recent weight loss, increased sweating, heat intolerance, hair loss, diarrhea, heart palpitations, anxiety and muscle weakness. Conversely, hypothyroidism can cause weight gain, cold intolerance, fatigue, dry skin, depression, change in menses and/or muscle cramps. Risk factors for TED such as smoking or a positive family history should also be assessed.
For those with known thyroid disease, history of planned, current and past therapies should be documented. This is especially important for those with a history of or planned radioactive iodine therapy, as this has been shown to exacerbate or trigger the onset of TED.
The clinical exam should be comprehensive, as TED can present with various clinical signs and symptoms. Patients may complain of dry eyes, pressure or pain behind the eyes and sandy or gritty sensations. Blurred vision, double vision and excessive tearing are also common. They may have an increase in redness or fullness to the eyelids. Some may even notice a change in their physical appearance and report that their eyes look bigger or that they just look different. Diurnal variation is common, with symptoms typically worse in the morning.
Assessment of the patient outside of the slit lamp can provide the first clue of thyroid dysfunction. Facial asymmetry can be subtle and is usually lost behind the magnification of the slit lamp. Attention should be given to the relative positions of the upper and lower eyelids. Upper eyelid retraction is one of the hallmark signs of hyperthyroidism, but it can oftentimes be subtle and overlooked.
This retraction is often characterized as having a “temporal flare,” where the lateral aspect of the lid margin is higher than the medial. Retraction can be due to one or a combination of factors including proptosis, levator/Müller’s muscle hypertrophy and increased sympathetic tone.6 The margin to reflex distance (MRD) can be used to quantify upper eyelid retraction. MRD1 is the distance from the pupillary light reflex to the upper eyelid margin. MRD2 is that to the lower eyelid margin. You can also measure the amount of “superior scleral show” or 12:00 limbus to upper eyelid margin at the slit lamp in primary gaze. A value greater than 5mm or asymmetry greater than 1mm is considered abnormal. Von Graefe’s sign (superior lid lag on downgaze) can also be observed in TED.
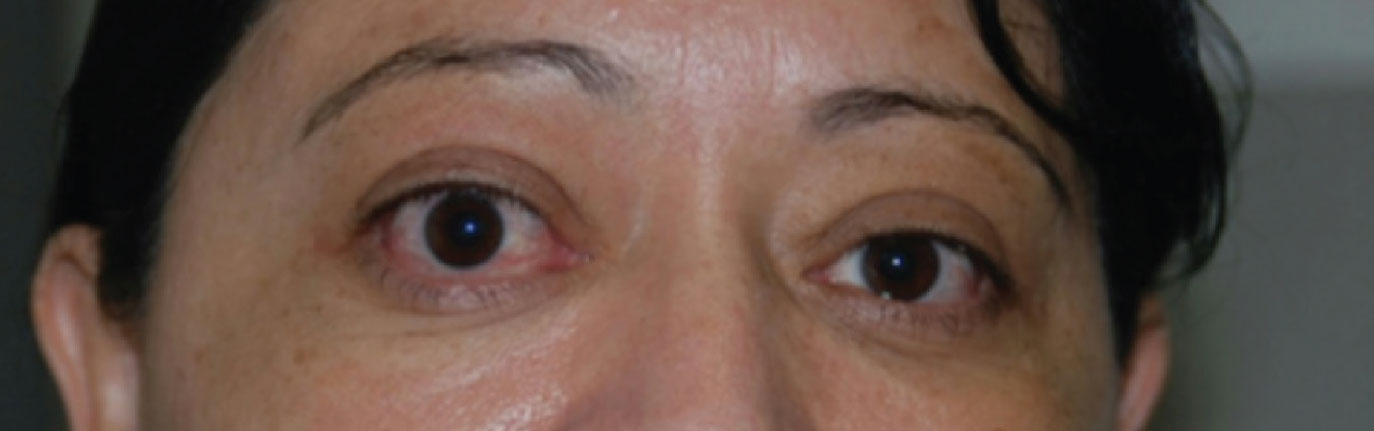 |
| Mild proptosis, upper lid retraction and conjunctival injection evident the right eye. Photo: Michael Richard, MD. Click image to enlarge. |
A basic sensory motor exam should always be performed. Extraocular motility and alignment defects can come from infiltration/inflammation of the extraocular muscles (EOMs). This most commonly involves the inferior and medial recti, followed by the superior and lateral recti and oblique muscles. Keep in mind that motility issues in TED are restrictive and do not improve with forced duction testing. This can be useful in differentiating TED from neurogenic causes such as a cranial nerve palsy or myasthenia gravis. A relative afferent pupillary defect is specific for optic nerve dysfunction but is not particularly sensitive when there is bilateral and symmetric disease.
Any patient suspected of having TED should be evaluated for proptosis. This can be easier to detect clinically from a “worm’s eye view,” with the patient chin-up or laid back while the clinician views their eyes tangentially from below. Displacement of the globe anteriorly can be measured with an exophthalmometer or by radiologic imaging. Hertel exophthalmometry is a quick, easy and reliable test that is found in most comprehensive eye clinics. Normal values vary by race and gender. For Asian patients, 16mm to 18mm is considered normal, whereas 18mm to 20mm is normal for Caucasian and 20mm to 22mm is normal for Black patients.7 Proptosis reduction can be a primary measure for treatment outcomes and should be recorded at baseline and follow-up visits consistently.
Anterior examination at the slit lamp can reveal evidence of inflammation of the conjunctiva with injection and chemosis. Swelling and injection of the caruncle and muscle insertions are also associated with TED. The tear film and cornea should be evaluated with fluorescein dye. A decreased tear meniscus, shorter tear breakup time and punctate epithelial erosions are suggestive of exposure issues. More advanced cases of TED can have corneal thinning and ulcerations.
The posterior exam should include careful assessment of the optic nerve. Approximately 3% to 7% of patients with TED will have optic neuropathy.8 The mechanism of DON is thought to be the result of inflammation, compression and ischemia. DON is rarely seen in Graves’ orbitopathy patients without significant muscle enlargement. In fact, medial rectus volume as measured with CT imaging has been shown to be the greatest predictor of DON.9
Since optic nerve compression usually occurs from congestion at the orbital apex, visible changes at the optic nerve head can be subtle or absent. Ancillary testing can help support the clinical suspicion of DON. Automated visual fields can show various defects, the most common being altitudinal, arcuate and paracentral.7 Color vision has been shown to be reduced in 77% of eyes with DON and should be assessed in patients suspected of having TED.10 More specifically, a 2021 study showed that a tritan (blue-yellow), rather than a protan (red-green) test, was more sensitive for DON.11
Studies looking at RNFL thickness have been conducted with variable results. One case-controlled observational study showed an overall increase in superior, nasal and inferior RNFL thickness values in TED patients compared with controls.12 A prospective longitudinal study showed that RNFL thinning can occur even in mild or subclinical TED.13 Both studies excluded those with clinically evident DON. The differences in outcomes of these studies can probably be explained by differences in methodology and patient demographics.
Furthermore, the pathogenesis of DON is believed to involve both compression and inflammation, the two of which will have different effects on RNFL thickness. Where active inflammation can increase RNFL thickness values, compression results in thinning of the RNFL. Several studies, including the aforementioned, have supported the finding that macular and ganglion cell layer-inner-plexiform layer thickness may be a more sensitive test for optic neuropathy in TED patients.14
Macular OCT can also be useful in identifying more subtle retinal changes that can occur with TED. For instance, choroidal thickness can vary in patients with TED.15 Macular OCT angiography has also been used to show changes in retinal and choroid blood flow.16,17 Chorioretinal folds caused by posterior flattening of the globe can be more easily visualized on OCT as well.
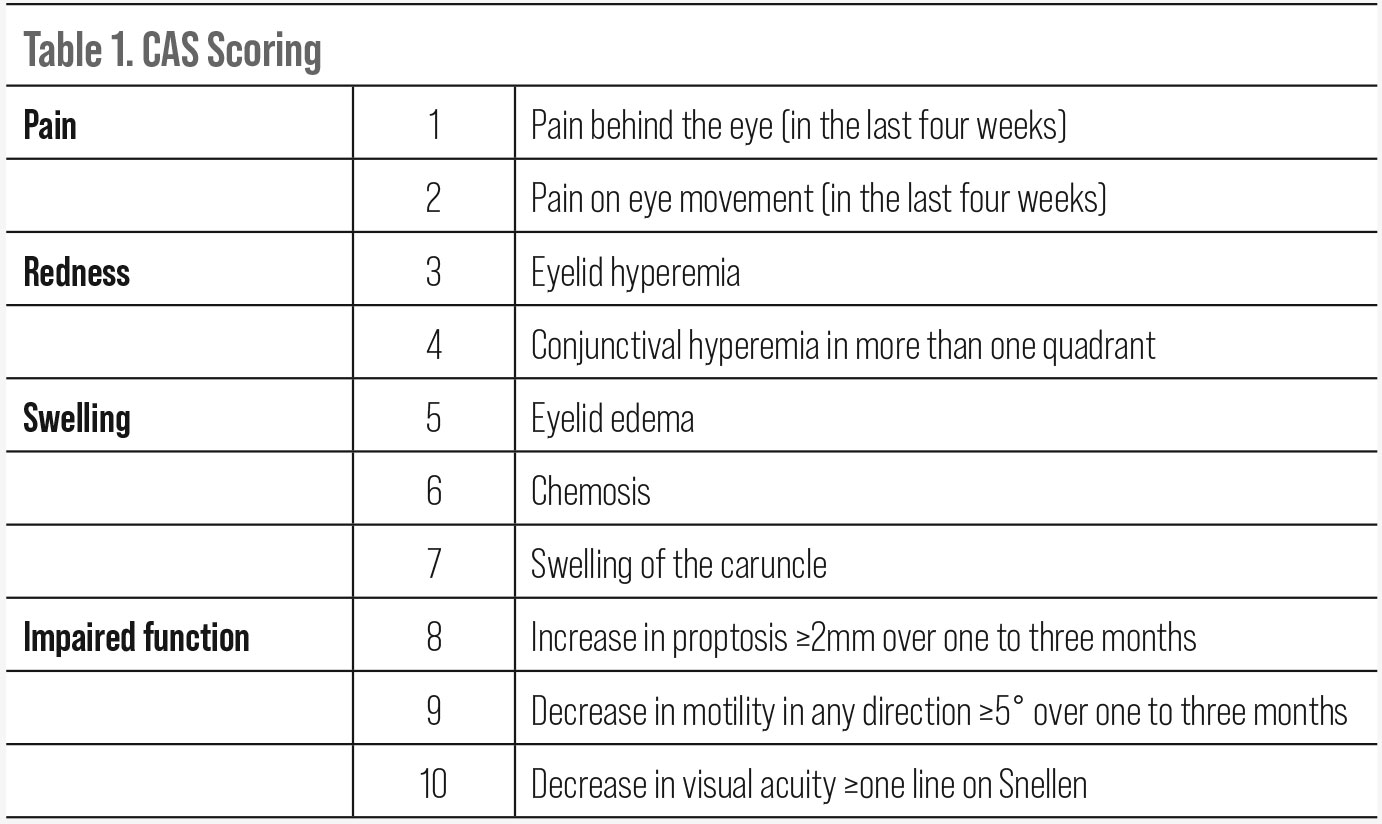 |
| Click table to enlarge. |
Making the Diagnosis
The diagnosis of TED can be made when two of three criteria are met: 1) clinical signs and symptoms as discussed previously, 2) laboratory tests and 3) radiologic signs consistent with thyroid orbitopathy.
Laboratory testing. The three primary tests used to assess thyroid function are TSH, T3 and FreeT4. With the vast majority of TED cases being secondary to GD, it’s helpful to include tests for thyroid-stimulating immunoglobulins (TSI) or TSH receptor antibodies. A thyroid antibody panel is sometimes available and will also include thyroid peroxidase antibodies that can be positive in Hashimoto’s disease. A strong correlation exists between TSI levels and TED activity scores. Monitoring with TSI levels can sometimes be useful when trying to determine a patient’s clinical activity.
Radiologic imaging. When unilateral, equivocal moderate-to-severe clinical disease or DON is suspected, orbital imaging is necessary. Although MRI may be better for soft tissue differentiation, non-contrast CT is preferred due to its lower cost, widespread availability and rapid acquisition time. In most cases, adequate assessment of the EOMs can be achieved without a contrast medium due to the vastly different densities of muscle and fat. CT also allows for the evaluation of the orbital walls and sinuses which can be helpful when planning orbital decompression surgery. MRI allows for better resolution of soft tissue and can be useful for cases where the clinical diagnosis is uncertain, atypical or unilateral. It may also be helpful in assessing clinical activity.
EOM belly enlargement (with tendon sparing) and fat expansion are the hallmark radiologic findings of Graves’ orbitopathy. The most common muscles involved are the inferior rectus and medial rectus, followed by the superior rectus, lateral rectus and oblique rectus. However, other findings such as fat stranding, anterior soft tissue swelling, lacrimal gland enlargement, exophthalmos and superior optic vein dilatation can also be observed.18
Clinical Grading Systems
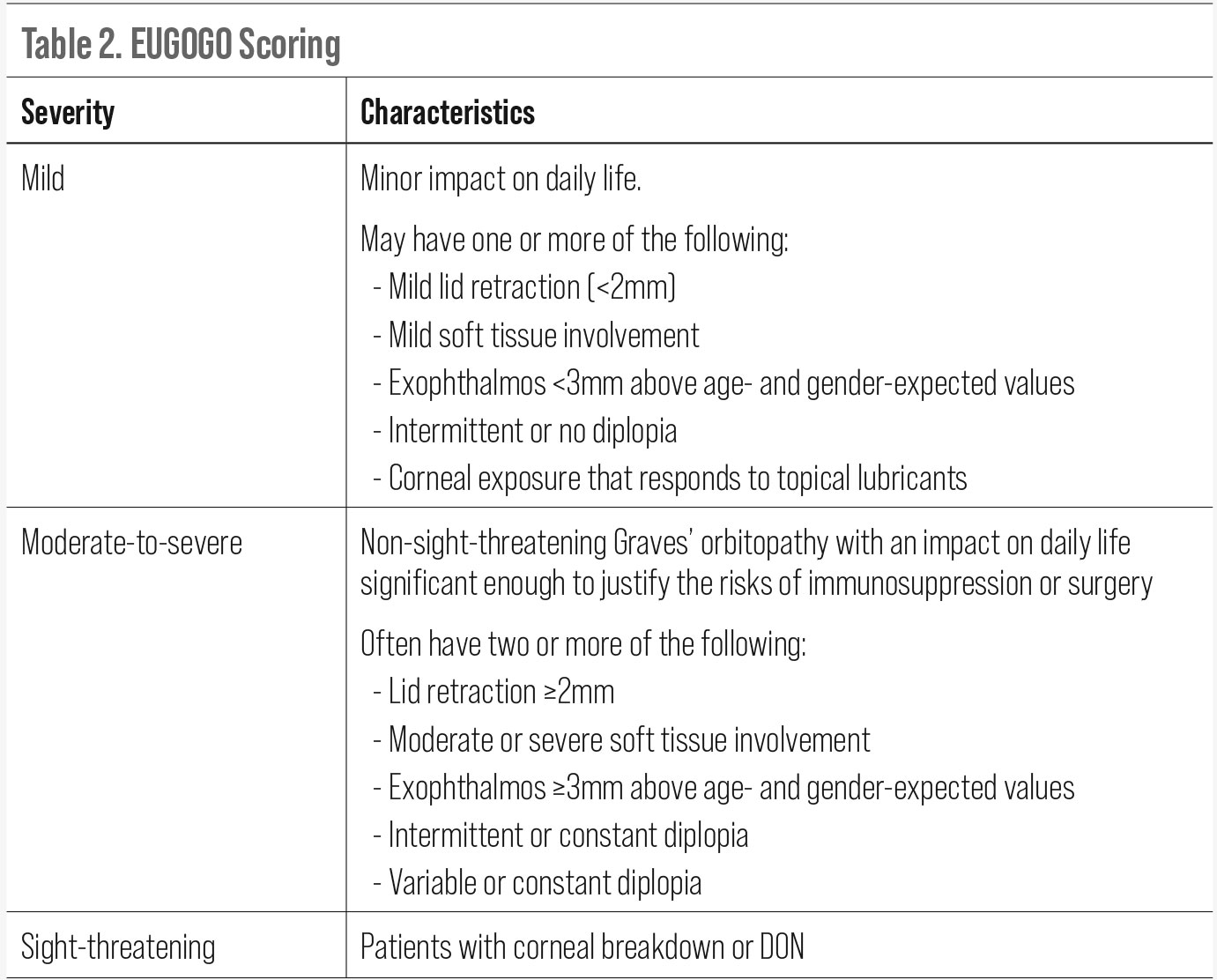
Management of TED is based on clinical disease activity and severity of signs and symptoms. Accurate assessment of these parameters is critical when developing a treatment plan. There have been a handful of grading systems that have been created over the years to facilitate this process. The most common are the CAS, NOSPECS, VISA and EUGOGO classification systems.
CAS, first published in 2003, stands for Clinical Activity Score and has been widely used in evaluating treatment efficacy in clinical trials. It is based on four classic signs of inflammation (pain, redness, swelling and impaired function). One point is given for each clinical element (one through seven) present the initial visit. Elements eight through 10 are used on follow-up to reflect disease progression. A CAS score of three or more is considered active disease. A score of four or more, when including elements eight through 10 at follow-up is indicative of progression.19
NOSPECS stands for No signs or symptoms, Only symptoms, Soft tissue involvement, Proptosis, EOM involvement, Corneal involvement and Sight loss. This system was developed in the late 60s and modified again in the late 70s. The mnemonic may be helpful to some clinicians or students as a reminder of the clinical signs and symptoms in TED, but its usefulness in monitoring progression and response to therapy is limited and has since fallen out of favor.
The VISA (Vision, Inflammation, Strabismus and Appearance) classification system was developed in 2006 and has been adopted by the International Thyroid Eye Disease Society (ITEDS). It attempts to quantify activity and severity levels while also making basic treatment recommendations. VISA uses a weighted point system to determine severity, and each parameter is graded independently. Activity is measured by progression within any of the categories as defined within each element. ITEDS has a clinical record form available online for download that can guide practitioners through the scoring and monitoring process. The VISA grading system incorporates both objective and subjective data to create its management plans.
The EUGOGO (European Group on Graves’ Orbitopathy) protocol was developed in 2008 and revised in 2016 and again in 2021.20 This is the most widely accepted system used today and attempts to provide grading of clinical activity and severity as well as provide guidance on treatment and management. It is the first system to include a GD-specific quality of life questionnaire (GO-QoL) in its algorithm. Disease activity scoring follows the CAS protocol where a score greater than three at the initial visit is active, and a score of four or higher at follow-up is considered active/progressive. Disease severity is broken down into three categories: mild, moderate-to-severe and sight-threatening.
 |
| Click table to enlarge. |
Patient Management
For all patients with GD, the first step in management is to control risk factors for progression. Management of thyroid disfunction is crucial and will involve communication with the patient’s primary care physician or endocrinologist. A referral to oculoplastics or neuro-ophthalmology should be made for those with clinically active moderate-to-severe or sight-threatening disease. A discussion about smoking cessation is imperative, as evidence suggests current smokers are twice as likely to develop TED and more likely to have severe disease and relapse than non-smokers.21
Mild disease. For mild Graves’ orbitopathy, management focuses on local treatment of the ocular surface. Dry eye treatment options are ever-expanding and are certainly in the wheelhouse of modern-day optometry. Ocular lubricants in the form of tears, gels and ointments are first-line options in exposure-related dry eye. Punctual occlusion can also be considered.
The role of topical immunomodulators like cyclosporin and lifitegrast has yet to be established in patients with active Graves’ orbitopathy. One study on topical cyclosporine showed no benefit to those with inactive TED and dry eye disease already taking artificial tears four times a day.22
Intermittent diplopia can sometimes be managed with prismatic correction. The use of fresnel prism may be more appropriate as the magnitude and direction can change frequently.
Selenium supplementation (200µg/day) has been shown to reduce progression of Graves’ orbitopathy and improve quality of life in those with mild disease; however, this benefit has only been shown in selenium-deficient areas.20 Selenium supplementation can help achieve euthyroidism faster in those with GD and inadequate intake. Laboratory testing of serum selenium levels prior to initiating supplementation is recommended so that overdosing and toxicity can be avoided.23
Sometimes mild inactive disease can lead to cosmetic disfigurement and quality of life issues that can justify a surgical referral. An example would be when there is unilateral lid retraction or proptosis that does not meet the moderate-to-severe criteria.
Moderate-to-severe disease. The EUGOGO 2021 publication of clinical practice guidelines is a comprehensive manual on the treatment of moderate-to-severe and sight-threatening Graves’ orbitopathy. Recommendations were made by a consensus of a 48-member task force, following a comprehensive review of published clinical trials. These recommendations will be reviewed later.
For active moderate-to-severe Graves’ orbitopathy, first-line treatment is often with systemic corticosteroids. Intravenous methylprednisolone (IVMP) is given at either an intermediate dose (500mg once weekly for six weeks followed by 250mg weekly for six weeks) or a high dose (750mg weekly for six weeks followed by 250mg weekly for six weeks). High-dose IVMP is reserved for more severe disease as it carries a higher risk of liver toxicity and cardiovascular side effects. Intravenous administration of steroids is preferred due to increased efficacy and overall fewer side effects when compared with oral steroids.20,24 A referral to an institution with the means and experience to manage dosing and side effects is suggested. The benefits of IVMP are maximized with earlier intervention, and delayed referral can affect clinical outcomes.
Contraindications for IVMP include recent hepatitis, liver dysfunction, cardiovascular morbidity, severe hypertension, inadequately managed diabetes and severe or uncontrolled glaucoma.25 Prior to starting a corticosteroid, control of diabetes and hypertension should be optimized and there should be extensive discussion on patient expectations and outcomes. Although clinical and quality of life improvement following steroid treatment can be as high as 80%, it is unlikely that patients return to their pre-disease state, and additional treatment and sometimes surgery is required to manage residual complications of Graves’ orbitopathy like strabismus and proptosis.
When there is an inadequate response to initial steroid therapy, secondary treatment options can be considered. Options are numerous, and decision-making is multifactorial.
Orbital radiotherapy is generally considered a safe and effective second-line therapy. It’s most beneficial in reducing soft tissue swelling and can improve extraocular motility; however, it does not typically improve proptosis. It is more effective when combined with oral or IV steroids.20 It should be avoided in patients with hypertensive or diabetic retinopathy due to the potential for radiation retinopathy.
There are several immune-modulators that have emerged in the last decade that can be used as an adjunct to steroids or sometimes as monotherapy. One of the more favorable is mycophenolate, a drug primarily used as an immunosuppressant following organ transplantation. Its popularity for use in other autoimmune disease has grown due to its safety and efficacy profile.24 Mycophenolate is known to inhibit proliferation of B- and T-cells as well as fibroblasts.
As an adjunct, it has been shown to increase the efficacy of IVMP without increasing side effects. Thus, mycophenolate has been recommended with IVMP as a first-line option by the EUGOGO. It can also be used as a standalone option when steroids are contraindicated.20 Mycophenolate sodium is taken orally at 0.72g/day.
Cyclosporine, another T-cell inhibitor, has been shown to be effective only in combination with steroids. When trialed as monotherapy, it was inferior to corticosteroids in both efficacy and relapse rate. The potential for renal toxicity makes cyclosporine less attractive than other second-line options. Azathioprine is another anti-proliferative agent that is similar to mycophenolate in mechanism but carries a higher risk of side effects.
The emergence of monoclonal antibody therapies (mAbs) has significantly changed the treatment landscape in nearly all areas of medicine over the last three decades. This class of medications can bind to their target with high specificity. Because mAbs do not undergo hepatic or renal metabolism, they also have a lower propensity for drug interactions. The evolution of mAbs from murine and chimeric antibodies to fully human mAbs has significantly helped to reduce the risk of developing an anti-antibody response.26
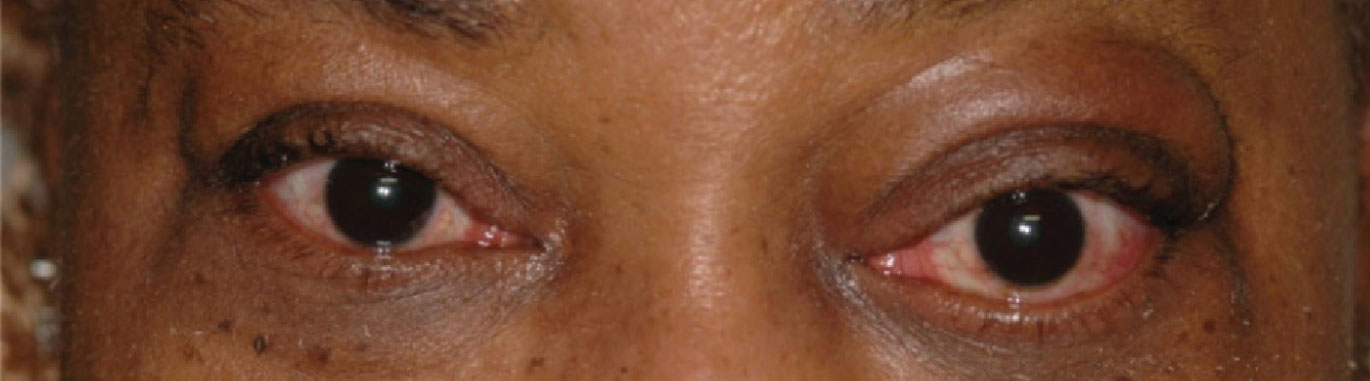 |
| Proptosis, eyelid edema, mild upper lid retraction and lower lid lagophthalmos. Photo: Michael Richard, MD. Click image to enlarge. |
A handful of mAbs have been studied in clinical trials. The most notable are rituximab, tocilizumab and teprotumumab. Rituximab is a chimeric mAb against the CD20 surface antigen found on B-cells. Few randomized controlled trials of rituximab in patients with Graves’ orbitopathy have been done, and the results have been conflicting.
Tocilizumab is a humanized mAb against the interleukin-6 receptor. Clinical trials with tocilizumab have only been done with steroid-resistant TED, and the results were favorable for CAS reduction and disease inactivation; however, proptosis reduction was modest, and there was no difference in quality of life measures compared with placebo.20,24
Teprotumumab, a fully human mAb, is the first FDA-approved treatment for Graves’ orbitopathy. It is directed against the IGF-1 receptor, which, as discussed earlier, is coupled with the TSH receptor and over-expressed by OF, T- and B-cells in GD. Phase III trials of teprotumumab (OPTIC trials) showed a significant number of patients had ≥2mm reduction in proptosis when compared with placebo (83% vs. 10%, respectively). Inactivation of disease was 59% in the treatment group vs. 21% in the placebo group. A significant reduction in diplopia was achieved in 68% vs. 29% of cases, respectively.27
A follow-up and extension trial (OPTIC-X) included participants from the OPTIC trial that either were non-responders, experienced relapse or belonged to the placebo group. Naturally, these patients had a longer mean duration of active disease than the original OPTIC study participants (12.9 months vs. 6.3 months). Patient outcomes and adverse events were similar to the original study and were validated over a longer follow-up period (48 weeks vs. 24 weeks in the OPTIC trial).
Results from the OPTIC-X trial suggest that response to treatment with teprotumumab is durable in most, and some non-responders can potentially benefit from a second treatment. It also raises the question of whether teprotumumab can be used in those with inactive disease, as a handful of patients who experienced proptosis reduction had a CAS score of zero or one at baseline.29
Teprotumumab is administered by infusion with a dose of 10mg/kg for the first dose, followed by seven infusions of 20mg/kg spaced three weeks apart for a total of 24 weeks. It is generally well-tolerated with most side effects being mild-to-moderate. The most common are muscle spasms, alopecia, nausea and fatigue. Less than 5% of patients in the clinical trials experienced hearing impairment and hyperglycemia. Patients with irritable bowel disease should be counselled, as a flareup is possible. Infusion reactions can occur and include transient increases in blood pressure, tachycardia, dyspnea, headache and muscular pain. Reactions are usually mild or moderate in severity and can usually be successfully managed with corticosteroids and antihistamines.27,28
Recommendations for use of teprotumumab in clinical practice were published by the OPTIC trial investigators in 2019.29 The authors suggest that prior to starting teprotumumab, patients should undergo a complete physical exam (with measurements of height, weight and blood pressure), standard laboratory tests (including complete blood count, liver function tests, fasting glucose and hemoglobin A1c) and an electrocardiogram. A comprehensive eye exam should be performed and include measurements such as motility, strabismus and exophthalmometry.
Teprotumumab has not been compared head-to-head with steroids or other mAbs in clinical trials. The biggest barrier to treatment currently is cost, especially when compared with corticosteroids. A 24-week course can run close to $350,000. Prior authorization is needed for most insurance plans, and some may require a trial with steroids prior to approval.
Sight-threatening disease. Vision loss from DON or severe corneal comprise occurs in a small percentage of patients with TED. Prompt referral is necessary to ensure optimal outcomes in these cases. Treatment of such sight-threatening disease usually involves a course of high-dose IVMP and is sometimes followed by orbital radiation and/or surgical decompression. Decompression is achieved by removing soft tissue and/or expanding the bony walls to decompress the optic nerve at the orbital apex.30 There are different approaches to decompression surgery, and the choice can vary by the patient’s needs and the surgeon’s preference. Teprotumumab or other immunologic agents may play a role in treating sight-threatening TED especially in refractory cases or when surgery may be contraindicated.
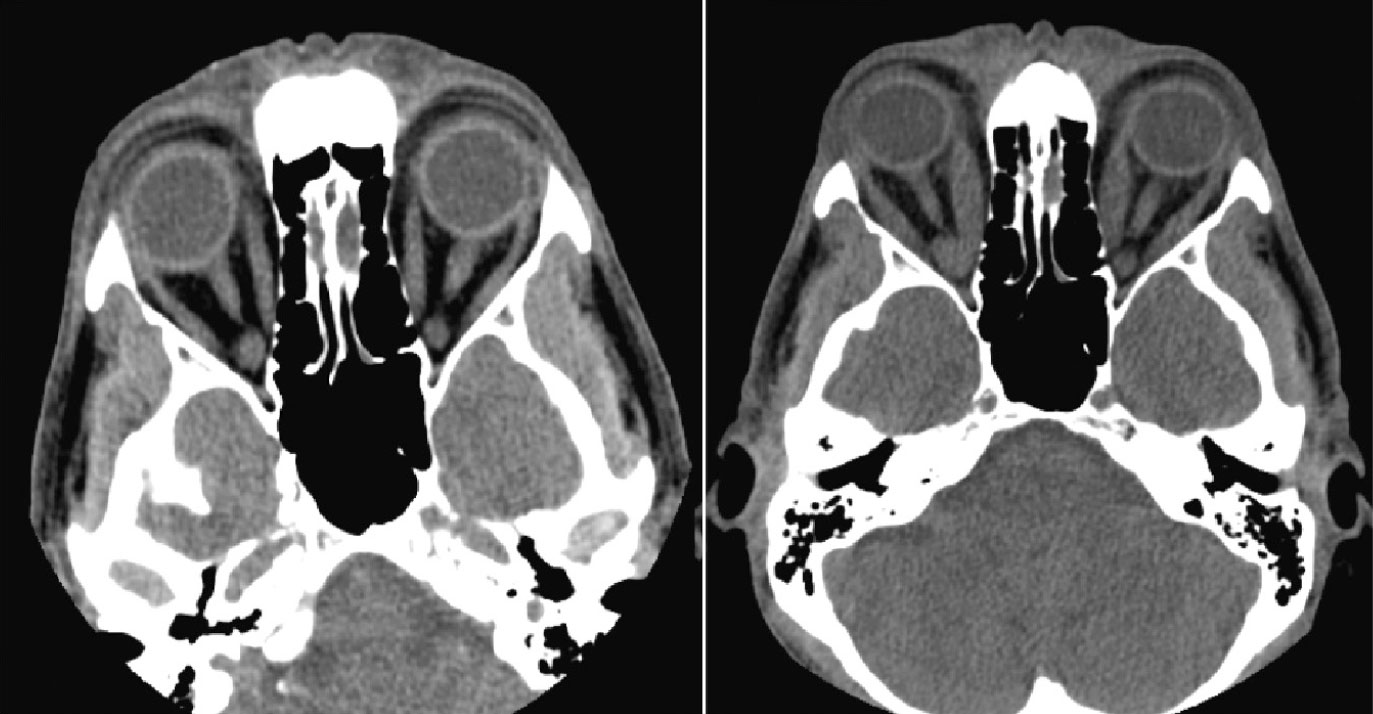 |
| CT of orbits showing a patient in 2017 before the onset of GD (left) and in 2020 after onset (right). Note the medial rectus enlargement. Click image to enlarge. |
Inactive disease. As noted, despite even the best attempts at controlling inflammation during the active phase of TED, very few patients return to their pre-disease state when it subsides. Dry eye, diplopia, lid retraction and proptosis are common sequelae. Some patients, especially those with concern for chronic exposure keratopathy and/or significant disfigurement, may require surgery. In extreme cases, multiple procedures may be necessary. Comanagement with strabismus and oculoplastic surgeons is not uncommon.
Patients may have to wait months for their disease to stabilize before they are eligible for surgery, and during that time temporary solutions may be necessary. For diplopia, Fresnel prism or patching may be needed. Taping lids and aggressively lubricating with gels and ointments can help protect the cornea from exposure. With or without rehabilitative surgeries, residual dry eye and diplopia can still occur. Various strategies exist in the management of these chronic conditions.
Summary
TED has the potential to be a sight-threatening and debilitating condition that can present with a wide variety of clinical signs and symptoms. It is characterized by an active phase of autoimmune driven orbital and soft tissue inflammation that lasts roughly six to 18 months in total, followed by an inactive phase of chronic tissue fibrosis. Disease relapse (or reactivation) is uncommon but not rare and may occur in about 15% of affected patients.30
A diagnosis of TED is made by a combination of clinical signs, laboratory testing and/or radiologic imaging. In order to optimize treatment outcomes, early recognition is necessary, and communication with primary care and/or endocrinology for the treatment of the underlying hyper or hypothyroidism is critical.
Clinical disease activity and severity grading should be assessed to help determine the best treatment strategy for the patient. Moderate-to-severe and sight-threatening active disease should be referred to subspecialists with experience treating TED. First-line treatment for these severity stages has historically involved intravenous steroids; however, newly developed and more targeted treatments are gaining popularity due to their potential for better clinical outcomes and safety profiles.
As a primary eyecare provider, a comprehensive understanding of the clinical signs of TED as well as the diagnostic testing and appropriate treatment and monitoring strategies is critical to ensure better outcomes for your patients with this condition.
Dr. Carstens is a clinical supervisor and director of optometric education at the Durham VA Medical Center in North Carolina. He has no relevant financial interests to disclose.
1. Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Ophthalmol. 1993;111(2):197-201. 2. Davies T, Burch H. Clinical features of Graves’ orbitopathy (ophthalmology). UpToDate. Last updated Mar 19, 2021. 3. Koumas L, Smith TJ, Feldon S, et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291-1300. 4. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. 2016;142:83-91. 5. Krieger CC, Boutin A, Jang D, et al. Arrestin-β-1 Physically Scaffolds TSH and IGF1 Receptors to Enable Crosstalk. Endocrinology. 2019;160(6):1468-79. 6. Small RG. Upper eyelid retraction in Graves’ ophthalmopathy: a new surgical technique and a study of the abnormal levator muscle. Trans Am Ophthalmol Soc. 1988;86:725-93. 7. Denisova K, Barmettler A. Evaluating the thyroid eye disease patient. Int Ophthalmol Clin. 2021;61(2):33-52. 8. Blandford AD, Zhang D, Chundury RV, et al. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. 2017;12(2):111-21. 9. Weis E, Heran MK, Jhamb A, et al. Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis. Ophthalmology. 2012;119(10):2174-8. 10. McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91(4):455-8. 11. Garip Kuebler A, Halfter K, Reznicek L, et al. A pathological indicator for dysthyroid optic neuropathy: tritan color vision deficiency. Graefes Arch Clin Exp Ophthalmol. 2021;259(11):3421-6. 12. Blum Meirovitch S, Leibovitch I, Kesler A, et al. Retina and nerve fiber layer thickness in eyes with thyroid-associated ophthalmopathy. Isr Med Assoc J. 2017;19(5):277-81. 13. Mugdha K, Kaur A, Sinha N, et al. Evaluation of retinal nerve fiber layer thickness profile in thyroid ophthalmopathy without optic nerve dysfunction. Int J Ophthalmol. 2016;9(11):1634-7. 14. Guo J, Li X, Ma R, et al. The changes of retinal nerve fibre layer and ganglion cell layer with different severity of thyroid eye disease. Eye (Lond). 2022;36(1):129-34. 15. Gul A, Basural E, Ozturk HE. Comparison of choroidal thickness in patients with active and stable thyroid eye disease. Arq Bras Oftalmol. 2019;82(2):124-8. 16. Del Noce C, Vagge A, Nicolò M, et al. Evaluation of choroidal thickness and choroidal vascular blood flow in patients with thyroid-associated orbitopathy (TAO) using SD-OCT and Angio-OCT. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1103-7. 17. Yu L, Jiao Q, Cheng Y, et al. Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography. BMC Ophthalmol. 2020;20(1):421. 18. Gonçalves AC, Gebrim EM, Monteiro ML. Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy. Clinics (Sao Paulo). 2012;67(11):1327-34. 19. Mourits MP, Prummel MF, Wiersinga WM, et al. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 1997;47(1):9-14. 20. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. 21. Jain AP, Jaru-Ampornpan P, Douglas RS. Thyroid eye disease: redefining its management-a review. Clin Exp Ophthalmol. 2021;49(2):203-11. 22. Altiparmak UE, Acar DE, Ozer PA, et al. Topical cyclosporine A for the dry eye findings of thyroid orbitopathy patients. Eye (Lond). 2010;24(6):1044-50. 23. Dharmasena A. Selenium supplementation in thyroid associated ophthalmopathy: an update. Int J Ophthalmol. 2014;7(2):365-75. 24. Khong JJ, McNab A. Medical treatment in thyroid eye disease in 2020. Br J Ophthalmol. 2021;105(3):299-305. 25. Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96(2):320-32. 26. Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies: what cardiologists need to know. Circulation. 2013;127(22):2222-30. 27. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N Engl J Med. 2020;382(4):341-52. 28. Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab Efficacy, Safety, and Durability in Longer-Duration Thyroid Eye Disease and Re-treatment: OPTIC-X Study. Ophthalmology. 2022;129(4):438-49. 29. Douglas RS, Wang Y, Dailey RA, et al. Teprotumumab in clinical practice: recommendations and considerations from the OPTIC trial investigators. J Neuroophthalmol. 2021;41(4):461-8. 30. Patel P, Khandji J, Kazim M. Recurrent Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg. 2015 Nov-Dec;31(6):445-8. doi: 10.1097/IOP.0000000000000371. PMID: 25621464. |
