Surgical Options for the Correction of Astigmatism
Today's sophisticated surgical tools and techniques offer dramatically improved outcomes over the imprecise early efforts of yesterday.
Release Date:
November 2016
Expiration Date:
November 1, 2019
Goal Statement:
Surgical correction of astigmatism has a promising future. This article discusses the improved strategies that are evolving in the areas of topography-guided laser procedures, ray tracing, patterned corneal collagen crosslinking and toric intraocular lenses.
Faculty/Editorial Board:
Kristen Brown, OD
Credit Statement:
This course is COPE approved for 1 hour of CE credit. COPE ID is 51565-RS. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
The author has no financial interest in any products mentioned in this article.
Eye care practitioners are familiar with corneal astigmatism, as this common refractive condition caused by unequal curvature along the two principal meridians greets us in the exam chair every day. Classified by axis, astigmatism is either with-the-rule (WTR), against-the-rule (ATR) or oblique; it can be further categorized as regular or irregular. These terms and their clinical manifestations are a part of routine practice; however, the advent of wavefront analysis in recent years has enhanced our understanding of irregular astigmatism with the classification of higher-order aberrations such as coma, trefoil and quadrafoil.1
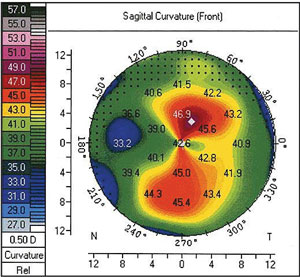 |
| Preoperative topography of a patient's left eye prior to having undergone a combined one-site cataract and trabeculectomy surgery with LRI. Regular 5.5D of with-the-rule astigmatism can be seen with an axis of 80°. Photo: Allister Gibbons, MD. |
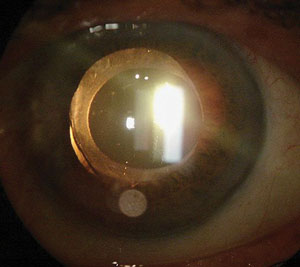 |
| The same patient three months after combined one-site cataract and trabeculectomy surgery with LRI. In retroillumination, we can verify the rotational stability of the lens at 80°. Photo: Allister Gibbons, MD. |
The portfolio of options for the surgical correction of astigmatism has expanded significantly over the past two decades. Photorefractive procedures such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) are well established. The introduction of femtosecond-laser (FS) technology has opened the door for new surgical strategies including laser-assisted cataract surgery and the recently FDA approved small-incision lenticule extraction (SMILE) procedure. Meanwhile, incisional options such as astigmatic keratotomy (AK) continue to evolve as FS technology adds a level of precision not seen with manual techniques. FS lasers can be combined with toric intraocular lenses (IOLs) to address the growing demand for refractive cataract surgery.
Surgical correction of astigmatism has a promising future. Improved strategies are evolving in the areas of topography-guided laser procedures, ray tracing, patterned corneal collagen crosslinking and toric multifocal intraocular lenses.2,3
The Rising Tide
Astigmatism accounts for about 13% of all refractive error.4 As much as 90% of the world's population has some degree of astigmatism, including 20% with 1.5D or more cylinder.5 Approximately 63% of individuals under 40 have 0.25D or more astigmatism, while most have less than 1D.6-8
Research reveals that myopia and astigmatism are increasing at an alarming rate, both surfacing at a younger age and growing in prevalence throughout the early and adolescent years.9,10 In the United States, myopia prevalence is nearly twice what it was 30 years ago.11-13 In 2015, investigators found that 50% of urban populations in East Asia were myopic, with 90% of university students displaying myopia or myopic astigmatism.10 Research estimates that myopia—and presumably myopic astigmatism—will affect nearly six billion people by the year 2050.13 This astonishing rise has ramifications for eye care professionals around the world involved in the prevention, management and surgical correction of myopia and astigmatism.
From AK to Smile: A Retrospective on Surgical Methodology
Astigmatic keratotomy was one of the first forms of refractive surgery developed to correct astigmatism. AK became available in the United States shortly after radial keratotomy (RK) was introduced in 1978.14 During AK, peripheral arcuate incisions are made with a diamond blade knife along the steep axis of corneal astigmatism to flatten that axis.15 Though RK has fallen out of favor due to diurnal fluctuation in acuity and unpredictable long-term results, AK is still a convenient, relatively inexpensive procedure. Compared with LASIK or PRK, however, astigmatic keratotomy is less predictable and has a lower range of astigmatic effect.16-19
The application of FS lasers has given surgeons better control of the length, depth and location of AK incisions, leading to finer precision, less risk and better visual outcomes.20-23 A big advantage of FS AK is that cuts can be penetrating or intrastromal without a break in the epithelium. This allows for titration of the effect and better patient comfort.24
Low to moderate astigmatism (1D to 3D) can also be corrected with intracorneal ring segments (ICRS). The goal of ICRS implantation is typically to delay or prevent corneal graft surgery; it is most often employed in ectatic conditions such as keratoconus, post-LASIK ectasia and pellucid marginal degeneration.25 As with AK, ICRS can be done manually or with an FS laser to create tunnels that are more precise, more predictable and associated with fewer complications and similar visual outcomes.26
Photoablation with an excimer laser (i.e., LASIK and PRK) has become the prevailing surgical option for patients who desire an alternative to eyeglasses and contact lenses. The FDA first approved the excimer for spherical myopia in 1995, but the option to treat astigmatism and hyperopia didn't become available until several years later. A 2004 study found that one to two million people in the United States undergo LASIK or PRK.27 US Military personnel opt for refractive laser surgery at twice the rate of the general population.28 Adverse events such as dry eye symptoms, night vision disturbances (ghosting, glare or halo) and residual refractive error are rare and most resolve after the first year.28 Customized ablation profiles materialized about a decade ago when aberrometers became available to measure the wavefront of the eye.
Wavefront data is obtained by projecting a flat plane or "wave" of light into the eye and measuring the location of the corresponding light spots reflected from the retina. The relative location of reflected light is then used to calculate the power of the optical system where each spot of light enters the eye. The goal of custom or wavefront-guided (WFG) laser ablation is to reduce the imperfections or higher-order aberrations (HOAs) of the optical system.29 Original "conventional" laser ablation profiles with the very first excimer lasers did not aim to correct HOAs and tended to create an oblate (centrally flat) corneal profile. They were also more likely to induce postoperative HOAs like spherical aberration. Wavefront-optimized (WFO) ablations were also designed to customize laser ablations but do not rely on wavefront measurement of the eye. WFO treatments are based on refraction data, keratometry and a peripheral ablation profile specific to each cornea. This peripheral algorithm results in a more prolate corneal shape following laser ablation and is thought to induce fewer postoperative HOAs.30
Custom ablations are indicated for myopia as high as -12D with astigmatism up to -3.75D on most wavefront-guided excimer laser platforms. Similarly, wavefront-optimized ablation profiles can correct up to -12D myopia and provide an expanded range of astigmatism correction—up to -6D, for example—with the Wavelight EX500 excimer laser (Alcon).
Wavefront-guided visual outcomes have been excellent compared with conventional ablation profiles; however, they are dependent of aberrometry measurements which are affected by pupil size, pupil centration and accommodation.31 In addition, corneal, lens and vitreous opacities limit the ability to capture good wavefront data. As lenticular aberrations change over time, correcting the wavefront of the whole eye may not be an optimal strategy for treating irregular astigmatism and other HOAs.
Topography-Guided Laser Ablation
Refractive surgeons in the United States can now perform topography-guided laser ablation, which been growing in popularity outside the United States for many years.32 The FDA granted approval for topography-guided treatment on normal (i.e., previously untreated) eyes on the Nidek laser in October of 2013 and the Alcon laser system in 2015.
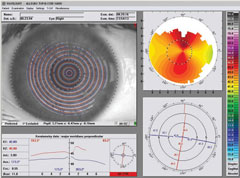 |
 |
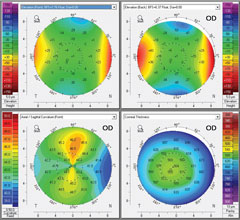
| Pre-op topography (Vario, Alcon) of a highly astigmatic patient to be treated with Wavelight FS200/EX500 (Alcon) wavefront optimized system. Left image: +4.25 -4.75x175 20/25 OD. Right image: +4.50 -5.25x005 20/25 OS. |
|
 |
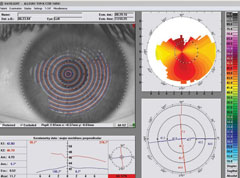
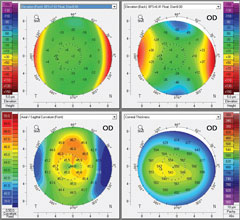 |
| Pentacam imaging of the same WFO-treated patient (right eye only). Left image: pre-op OD. Right image: one month post-op OD. |
|
 |
|
| At one month post-op, UCVA is 20/20 plano -0.25x005 20/20 OD. |
|
Topography-guided ablation normalizes or smooths the corneal surface by correcting only the HOAs that arise from the cornea, leaving internal aberrations unchanged. Topography-guided technology integrates placido disc topography, keratometry and pupillometry in a single diagnostic device that integrates with planning software and excimer laser technology.32 Unlike with WFG ablations, information about sphere and regular astigmatism (the lower-order aberrations) is not captured by topography and must be obtained separately, by refraction, for example.
Recent research evaluating the safety and effectiveness of topography-guided LASIK found that visual symptoms such as glare, halo and starbursts were reduced—and, more surprisingly, 30.9% of subjects gained one or more lines of uncorrected visual acuity compared with preoperative best corrected visual acuity.32 Outside the United States, topography-guided procedures can be conducted on highly aberrated eyes such as those with small or decentered optical zones from previous corneal refractive surgery, eyes with naturally occurring irregular corneal astigmatism or irregular astigmatism from trauma.33,34
With age, the axis of astigmatism shifts toward an ATR orientation primarily due to changes in the cornea, not the lens. A reduction in eyelid tension over time may partly explain this shift, as internal astigmatism remains relatively stable with age.35
Despite minimally invasive approaches, modern cataract surgery can induce corneal astigmatism even with the small incisions made in the peripheral cornea. Like AK, incisions made for cataract surgery entry wounds cause flattening in the meridian incised and steepening in the meridian 90 degrees away.36 Superior incisions cause flattening of the vertical meridian or an increase in ATR astigmatism, while temporal incisions lead to flattening in the horizontal meridian or an increase in WTR astigmatism. The amount varies with individual differences in corneal biomechanical properties, surgical technique and type of surgery performed.37 Understanding and managing surgically induced corneal astigmatism (SICA) is necessary for surgeons to succeed in an era of patients opting for refractive cataract surgery, which includes post-op uncorrected emmetropia as a goal. Many have had previous LASIK or PRK and expect to return to post-laser, spectacle-free status.
LRIs and IOLs
During cataract surgery, corneal astigmatism can be corrected with limbal relaxing incisions (LRIs), toric IOLs or a combination of the two. In addition, a femtosecond laser can be employed to assist the surgeon with steps that are typically performed manually. Laser-assisted cataract surgery (LACS) adds a level of precision to the procedure, which may improve visual outcomes and lessen the risk of postoperative complications such as endothelial cell loss and macular edema.38 LRIs are positioned more peripherally in the cornea than AK incisions and are preferred in cataract surgery since the same incision can be used for phacoemulsification. Compared with astigmatic keratotomy, LRIs are associated with less pain and less induced irregular astigmatism.39 Further, they are quick and effective at reducing up to 1.5D of corneal astigmatism.
Toric IOLs are a better option for surgical correction of moderate (1.5D to 4.25D) astigmatism. Several toric IOLs of various material and astigmatic power are available in the United States, including the Staar Toric (Staar Surgical), Acrysof IQ Toric (Alcon), Tecnis Toric (AMO) and Trulign Toric (Bausch + Lomb). For successful correction of astigmatism, toric IOLs must be aligned with the visual axis and the steep meridian of the cornea. Some lenses can rotate, which will reduce the effectiveness and leave residual astigmatism at a new, often oblique, meridian.
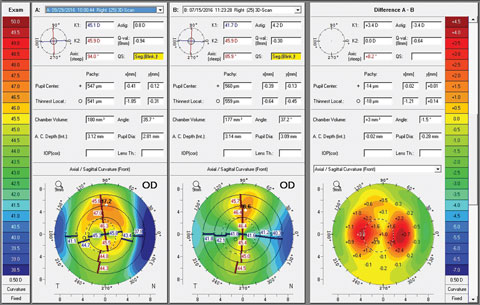
Anterior and posterior corneal astigmatism must both be considered when planning IOL surgery—particularly in patients who want less dependence on spectacle correction. Inaccuracies arise when posterior corneal astigmatism is measured with the assumption that there is a fixed-ratio relationship with the anterior corneal curvature.40 The Cassini Corneal Shape Analyzer (i-Optics) is a new topographer that uses LED ray tracing technology with 700 diode lights to measure anterior and posterior corneal astigmatism. Advances in cylinder and axis measurement precision can be useful for preoperative planning of toric IOL implants, particularly in post-laser refractive surgery patients.41
In addition, the use of intraoperative aberrometry can guide IOL power selection. The Optiwave Refractive Analysis system (Alcon) employs wavefront interferometry to produce a fringe pattern; distortions in this pattern are then translated into refractive values.42 Research on toric IOL power selection aided by intraoperative aberrometry reveals that patients were 2.4 times more likely to have less than 0.50D of residual refractive astigmatism when an intraoperative aberrometer was used.43
Advantages of LACS over manual cataract surgery include the customization of corneal incisions, precision and the centration of the capsulotomy for improved refractive stability and predictability. Fragmenting the lens before phacoemulsification is another advantage of LACS that is believed to be less likely to induce endothelial cell loss.43 For astigmatism greater than 4.25D at the corneal plane, it may be necessary to combine LRIs with toric IOLs or subsequent LASIK or PRK for up to 10D corneal astigmatism correction.
The newest frontier combines astigmatism and presbyopia treatment in one IOL. The recently FDA approved Tecnis Symfony Toric IOL (AMO) and the forthcoming Acrysof IQ Restor Toric (Alcon) are two early entrants into this emerging category.
No Longer an Afterthought
Although significant astigmatism is typically defined as 1D or more, amounts as low as 0.25D can impact visual acuity or visual quality in some individuals. Planning and predicting the outcome of refractive surgery is the foremost challenge a surgeon faces. With the explosion of more advanced technology and improved surgical outcomes, there is increasing expectation that refractive procedures can and will provide excellent visual results with minimal risk, and that astigmatism correction must now be part and parcel of the surgical approach. Future advances will focus on corneal biomechanical properties and wound healing response, which is one of the few remaining limitations affecting the predictability and stability of refractive surgery.
Dr. Brown is clinical director of TLC Laser Eye Centers of Boston, MA, and Providence, RI. She is a member of the TLC Clinical Director Advisory Group and holds a faculty appointment at the New England College of Optometry. She is involved in clinical research, teaching and lecturing.
1. Kelly JE, Mihashi T, Howland HC. Compensation of corneal horizontal/vertical astigmatism, lateral coma and spherical aberration by internal optics of the eye. J Vision. 2004;4:262–71.
2. Cummings AB, Kelly GE. Optical ray tracing-guided myopic laser in situ keratomileusis:1-year clinical outcomes. Clinical Ophthalmology. 2013;7:1181–91.
3. Ibrahim Seven MS, Sinha Roy A, Dupps WJ Jr. Patterned corneal collagen crosslinking for astigmatism: Computational modeling study. J Cataract Refract Surg. 2014 June;40(6):943–53.
4. Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90(1):5–19.
5. Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am A. 2001;18:1793–1803.
6. Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, et al. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35:70-5.
7. Saunders H. Age-dependence of human refractive errors. Ophthalmic Physiol Opt. 1981;(1):159–74.
8. Fledelius HC, Stubgaard M. Changes in refraction and corneal curvature during growth and adult life. A cross-sectional study. Acta Ophthalmol. 1986;64:487–91.
9. Gwizada J, Grice K, Held R, et al. Astigmatism and the development of myopia in children. Vision Research. 2000;40:1019–26.
10. Smith MJ, Walline JJ. Controlling myopia progression in children and adolescents. Adolesc Health Med Ther. 2015;6:133-40.
11. Read SA. Ocular and environmental factors associated with eye growth in childhood. Optom Vis Sci. 2016;93:1031–41.
12. Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–85.
13. Holden BA, Fricke TR, Wilson DA. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016 May;123(5):1036-42.
14. Waring GO. Refractive keratotmy for myopia and astigmatism. St Louis, MO: Mosby-Year Book; 1992.
15. Hanna KD, Jouve FE, Waring GO 3rd, Ciarlet PG. Computer simulation of arcuate keratotomy for astigmatism. Refract Corneal Surg. 1992 Mar-Apr;8(2):152-63.
16. Waring GO 3rd, Lynn MJ, McDonnell PJ. Results of the prospective evaluation of radial keratotomy (PERK) study 10 years after surgery. Arch Ophthalmol. 1994;112(10):1298-308.
17. Kemp JR, Martinez CE, Klyce SD, et al. Diurnal fluctuations in corneal topography 10 years after radial keratotomy in the Prospective Evaluation of Radial keratotomy Study. J Cataract Refract Surg. 1999 Jul;25(7):904-10.
18. Kohnen T, Buhren J. Corneal first-surface aberration analysis of the biomechanical effects of astigmatic keratotomy and a micro-keratome cut after penetrating keratoplasty. J Cataract Refract Surg. 2005;31:185–9.
19. Montes-Mico R, Munoz G, Albarran-Diego C, et al. Corneal aberrations after astigmatic keratotomy combined with laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:1418–21.
20. Abbey A, Ide T, Kymionis GD, Yoo SH. Femtosecond laser-assisted astigmatic keratotomy in naturally occurring high astigmatism. Br J Ophthalmol. 2009;93(12):1566–9.
21. Hoffart L, Proust H, Matonti F, et al. Correction of post-keratoplasty astigmatism by femtosecond laser compared with mechanized astigmatic keratotomy. Am J Ophthalmol. 2009;147(5):779–87.
22. Oshika T, Shimazaki J, Yoshitomi F, et al. Arcuate keratotomy to treat corneal astigmatism after cataract surgery: a prospective evaluation of predictability and effectiveness. Ophthalmology. 1998;105(11):2012–6.
23. Aristeidou A, Taniguchi EV, Tsatsos M, et al. The evolution of corneal and refractive surgery with the femtosecond laser. Eye and Vision. 2015 Jul 14;2:12.
24. Bahar I, Levinger E, Kaiserman I, et al. IntraLase-enabled astigmatic keratotomy for post-keratoplasty astigmatism. Am J Ophthalmol. 2008;146(6):897–904.
25. Cosckunseven E, Kymionis GD, Tsiklis NS, et al. One-year results of intrastromal corneal ring segment implantation (KeraRing) using femtosecond laser in patients with keratoconus. Am J Ophthalmol. 2008;145(5):775–9.
26. Piñero DP, Alio JL, El Kady B, et al. Refractive and aberrometric outcomes of intracorneal ring segments for keratoconus: mechanical versus femtosecond-assisted procedures. Ophthalmology. 2009;116(9):1675–87.
27. Duffey RJ, Leaming D. Trends in refractive surgery in the United States. J Cataract Refract Surg. 2004;30(8):1781–5.
28. Blitz JB, Hunt DJ, Cost AA. Post Refractive surgery complications and eye disease, active duty US armed forces 2005-2014. MSMR. May 2016;23(5).
29. Subbaram MV, MacRae SM. Customized LASIK treatment for myopia based on preoperative manifest refraction and higher order aberrometry: the Rochester nomogram. J Refract Surg. 2007 May;23(5):435-41.
30. Randleman JB et al. Outcomes of wavefront-optimized surface ablation. Ophthalmology 2007 May;114(5):983-8.
31. Sáles CS, Manche EE. One-year outcomes from a prospective, randomized, eye-to-eye comparison of wavefront-guided and wavefront-optimized LASIK in myopes. Ophthalmology. 2013 Dec;120(12):2396-402.
32. Stulting RD, Fant BS, The T-CAT Study Group. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. Cataract Refract Surg. 2016 Jan;42(1):11-8.
33. Alio JL, Belda JI, Osman AA, Shalaby AMM. Topography-guided laser in situ keratomileusis (TOPOLINK) to correct irregular astigmatism after previous refractive surgery. J Refract Surg. 2003;19:516–27.
34. Lin DT, Holland S, Tan JC, Moloney G. Clinical results of topography-based customized ablations in highly aberrated eyes and keratoconus/ectasia with cross-linking. J Refract Surg. 2012;28(11 suppl):S841-8.
35. Anstice J. Astigmatism—Its components and their changes with age. Am J Optom Arch Am Acad Optom. 1971;48:1001–6.
36. Simsek S, Yaşar T, Demirok A, et al. Effect of superior and temporal clear corneal incisions on astigmatism after sutureless phacoemulsification. J Cataract Refract Surg. 1998;24:515–8.
37. Dupps WJ Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83(4):709-20.
38. Callou TP, et al. Advances in femtosecond laser technology. Clinical Ophthalmology 2016:10 697–703.
39. Settas G, Settas C, Minos E, et al. Photorefractive keratectomy (PRK) versus laser assisted in situ keratomileusis (LASIK) for hyperopia correction. Cochrane Database Syst Rev. 2012;(6): CD007112.
40. Koch DD. The posterior cornea: hiding in plain sight. Ophthalmology. 2015;122(6):1070–1.
41. Kanellopoulos J, Asimellis G. Distribution and repeatability of corneal astigmatism measurements (magnitude and axis) evaluated with color light emitting diode reflection topography. Cornea. 2015;34(8):937–44.
42. Huelle JO, Katz T, Druchkiv V, et al. First clinical results on the feasibility, quality and reproducibility of aberrometry-based intraoperative refraction during cataract surgery. British J Ophthalmol. 2014;98(11):1484-91.
43. Hatch KM, Woodcock EC, Talamo JH. Intraocular lens power selection and positioning with and without intraoperative aberrometry. J Refract Surg. 2015;31(4):237–42.
