Ocular Health: A Matter of the Heart
Vascular dysregulation can have a huge impact on the eyes. Here is what you need to know.
By Candice Tolud, OD, and Joy Harewood, OD
Release Date:
October 1, 2015
Expiration Date:
October 1, 2018
Goal Statement:
Clinicians should always look at the eye as an extension of the cardiovascular system. Learning more about how vascular dysregulation affects the eye will alter our treatment approach to various ocular diseases. This article reviews vascular dysregulation, its ocular manifestations, its relationship to common systemic diseases, and the use of cardiovascular medications to better treat ocular disease.
Faculty/Editorial Board:
Candice Tolud, OD, and Joy Harewood, OD
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID 46877-SD. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint sponsored by the University of Alabama School of Optometry.
Disclosure Statement:
Drs. Tolud and Harewood have no financial relationships to disclose.
It has been said that the eye is the window to the soul. Maybe so, but it's the state of the heart that's often revealed in the presence of ocular disease. New insights into the pathogenesis of optic neuropathies and retinal vascular disease highlight the importance of having a multidisciplinary approach to comprehensive eye care.
Of particular interest is vascular dysregulation syndrome. Many studies over the years have described a potential link between vascular dysregulation and the eye.1-5 As our understanding of its ocular effects increases, the treatment options for various conditions are expanding.
This article will review vascular dysregulation, its ocular manifestations and its relationship to common systemic diseases. Finally, it will discuss the use of medications that traditionally support cardiovascular health to better treat ocular disease.
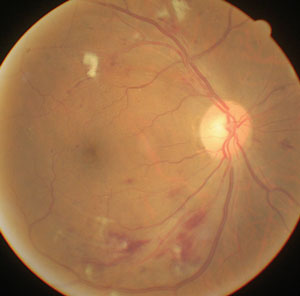 |
| Significant hypotensive retinopathy shows cotton-wool spots, striated retinal hemorrhages and narrowed retinal arterioles. Photo: Carlo Pelino, OD, and Joseph J. Pizzimenti, OD. |
Vascular Dysregulation
Inappropriate constriction or dilation of arteries, veins or capillaries in a particular tissue in the body is classified as either primary vascular dysregulation syndrome (PVD) or secondary vascular dysregulation syndrome (SVD).1 PVD derives from a process endogenous to the vasculature while SVD describes dysregulation due to an underlying non-vascular systemic disease.
Normal fluctuations in blood flow occur with activities of daily living. Blood flow varies due to changes in our natural circadian rhythm, alterations in our mental and physical activity and even simple changes in body position.
Patients with PVD respond abnormally to regular stressors like positional changes, coldness and other stimuli. Risk factors for PVD include female sex, slim build and Asian origin.6 The pathogenesis of PVD has yet to be determined. Affected patients are usually asymptomatic until they are exposed to one of the aforementioned stressors.
Classic symptoms of PVD include acute sensitivity to cold, reduced feeling of thirst, falling asleep, tinnitus, sudden hearing loss and a predisposition to migraines. The main signs include arterial hypotension, cold extremities and an increased sensitivity to cold. Other signs include low blood pressure (BP) and silent myocardial infarction. Upregulation of factor endothelin (ET-1) is also found and has specific effects on the eye (see "Secondary Vascular Dysregulation," below).
In a healthy individual, ocular blood flow is autoregulated by the local vasculature. Autoregulation is the eye's intrinsic ability to maintain blood flow despite changes in perfusion pressure. The vascular endothelium, neural and glial cells are responsible for much of this regulation.2 The vessels in the eye constrict and dilate to maintain a constant flow of blood and nutrients, thus maintaining ocular perfusion pressure (OPP) within a certain range.
Perfusion pressure in the body is the difference between arterial and venous pressures. OPP is calculated as the difference between retinal arterial pressure and intraocular pressure (IOP).7 Retinal arterial pressure can be measured using ophthalmodynamometry, during which pressure is applied to the side of the globe with a ophthalmodynamometer while observing the central retinal artery with an ophthalmoscope.8 The point at which pulsations are eliminated is the retinal arterial pressure. This measurement can also be approximated using the following formula: mean retinal arterial pressure = diastolic BP + 1/3(systolic BP – diastolic BP).9 Retinal venous pressure is thought to be well approximated by the IOP. This approximation is not always accurate, but widely accepted in the field.
Review of Ocular Vasculature
Due to the high oxygen demand of the retina, the choroid has the highest blood flow per volume in the body. |
PVD affects the eye by reducing ocular blood flow, which causes stiff retinal blood vessels, thus reducing the integrity of the blood/brain barrier and creating dysfunctional ocular autoregulation. These factors exacerbate pre-existing systemic conditions and their effect on the eye, as well as increase the risk of developing numerous primary ocular diseases. PVD is an independent risk factor for glaucoma, and those affected are more predisposed to retinal artery and vein occlusions and central serious chorioretinopathy.1,4-6,10-13 Patients with PVD and glaucoma may have a tendency to progress despite having normal IOP.11
Hypotension
Hypotension, defined as blood pressure below 90/60mm Hg, can result from low cardiac output, excessive blood pressure-lowering medications, postoperative complications, poor body positioning, or conditions such as anemia or congestive heart failure. Nocturnal hypotension is of particular interest to eye care providers because of its effect on the posterior segment. It can reduce perfusion to the eye, resulting in changes to both the optic nerve and the retinal vasculature.
Treatment for hypotension is aimed at the cause of low pressure. If it is a result of a surgical procedure, supplemental oxygen is typically given and the infusion rate is increased to combat hypovolemia, or low blood volume. Trendelenburg positioning, where the feet are elevated above the head, can help.14
• Hypotensive effects on optic nerve head perfusion. Ischemic disorders of the optic nerve head (ONH) are multifactorial in nature. Certain risk factors act in combination, with some simply predisposing the optic nerve to ischemia, while others are directly responsible for the final insult that produces ONH ischemia.4
While untreated hypertension poses it own risks (see "Hypertension," below), over-treatment of systemic hypertension can be deleterious as well. Although not unique to BP treatment, the J-curve phenomenon is seen in aggressive treatment of hypertensive patients when blood pressure is lowered so much that the beneficial effects of therapy are lost and the incidence of adverse events increases.4,15
Researchers speculate that the J-curve phenomenon is an important factor in ONH and ocular ischemic disorders.4,11 When systemic BP falls below the threshold necessary for the eye to maintain autoregulation, OPP drops, resulting in death of retinal ganglion cells and vision loss.4,11,15 Optic nerves experiencing the J-curve phenomenon are particularly susceptible during sleep hours when BP is at its lowest.4,12
Investigators have hypothesized that nocturnal hypoperfusion of the optic nerve is the precipitating factor in ONH ischemia in susceptible patients.4,11 As such, there is a significant association seen between progressive visual field loss and nocturnal hypotension in patients on oral hypotensive therapy (Figure 1).4
Fig. 1. Mechanism of ONH Ischemia Due to Nocturnal Hypotension in Predisposed Eyes:4 Decrease in BP during the night |
• Hypotensive effects on glaucoma. The pathogenesis of glaucomatous optic neuropathy is classified into the mechanical theory and the vascular theory. The former is well-documented: Increased IOP over time causes interruption of axoplasmic flow and subsequent death of optic nerve fibers.3,12 The vascular theory focuses on the potential development of intraneural ischemia resulting from decreased optic nerve perfusion.4
Much evidence suggests that vascular pathologies play an important role in the etiology and progression of both open angle glaucoma (OAG) and normal tension glaucoma (NTG).12 Low systemic BP favors the occurrence of visual field defects or development of greater visual field loss at any intraocular pressure, even in non-glaucoma subjects.4,16 Additionally, vascular insufficiency is considered even more severe in patients with NTG.16
NTG is a multifactorial optic neuropathy, and insufficient vascular autoregulation is considered to be present in at least some patients.16,17 Low BP, excessive BP dips and low ocular perfusion pressure are linked to NTG pathology and disease progression.11 Population-based epidemiological studies have shown that low diastolic BP and low diastolic OPP are major risk factors for NTG. Additionally, increased variability of mean arterial pressure over 24 hours is a risk factor for NTG development and progression.16
Lowering IOP in NTG patients is beneficial in preventing progression, as found in both the Collaborative Normal-Tension Glaucoma (CNTG) Study and the Early Manifest Glaucoma Trial.18,19 Prostaglandins are typically used as first-line therapy in NTG.18 The CNTG's recommendation for treatment of NTG was a 30% reduction in IOP.18,19 Interestingly, a more recent study found that brimonidine 0.2% BID, compared with timolol 0.5% BID, had less progression of visual field loss.15 Additionally, laser trabeculoplasty has also been shown to be effective in the treatment of NTG.20
• Non-IOP medical interventions. Although IOP plays a major role in both OAG and NTG, IOP-independent factors—such as oxidative stress, glutamate toxicity and vascular factors—still play important roles in the development of primary open-angle glaucoma (POAG) and NTG.12 As such, studies have explored treatments other than topical ocular hypotensives for their effectiveness in treating glaucomatous optic neuropathy.11,21 This is of particular interest for NTG patients who show disease progression in spite of normal IOP, decreased IOP or both.
Some research suggests that therapeutic increases in blood pressure in NTG patients may help increase ocular perfusion pressure.22 Investigators have studied several categories of drugs, including nitric oxide synthase inhibitors, antioxidants, excitotoxins, calcium channel blockers and nerve growth factors (Table 1). Research has shown that calcium channel blockers have favorable and significant effects on visual field and optic nerve fiber progression in NTG patients in a manner that was not equally seen in patients with POAG.11
Recent research has also shown that free radical scavengers such as ginko biloba extract (GBE) and anthocyanins might be effective in NTG.23 A small sample study showed that GBE increased the end-diastolic velocity in the ophthalmic artery without changes in arterial blood pressure, heart rate or IOP.24
• NAIONs. Non-arteritic ischemic optic neuropathy (NAION) results from ischemia of the retrolaminar part of the optic nerve head.25 Classically, it causes painless loss of vision or visual field, with more than 75% of patients noticing vision loss upon waking.21 NAION is the most common acute optic neuropathy in people over 50.26
| Table 1. Topical IOP-mediated and Oral Non-IOP Drugs: Effects on Ocular Perfusion1,2 | ||
| Potential Medications for Increased Blood Perfusion | Proposed Mechanism of Action | |
| Topical Medications |
||
| Calcium channel blockers |
Less progression of visual field |
|
| Betaxolol |
Improved choroidal flow, better visual field preservation |
|
| Dorzolamide |
Increased retinal blood flow velocity in humans |
|
| Brimonidine |
Increased retinal ganglion cell survival in rat optic nerve crush injury |
|
| Non-IOP Mediated Oral Medications |
||
| N-methyl-D-aspartate (NMDA) receptor antagonist (Memantine) |
Prevents binding of glutamate and resultant calcium influx; blocks rat ganglion cells from glutamate toxicity and blocks toxic level of glutamate in vitreous |
|
| Serotonin S2 receptor antagonist (Naftidrofuryl) |
Arteriolar vasodilation, improved blood flow in Raynaud syndrome |
|
| Free radical scavengers - Ginkgo biloba |
Scavenges free radicals and nitric oxide, improves blood flow |
|
| 1. Netland PA, Chaturvedi N, Dreyer EB. Calcium channel blockers in the management of low-tension and open-angle glaucoma. Am J Ophthalmol. 1993 May;115(5):608-13. 2. Freudenthal J et al. Low-tension glaucoma medications. Medscape. 2014 Oct. www.emedicine.medscape.com/article/1205508-medication. |
||
Acutely, optic disc swelling and hemorrhages with pallor of the disc develop over time.4,21 Research has also noted that some discs have certain anatomic features that seem to predispose them to NAION, referred to as "discs at risk."4,21,27,28 These features include a small nerve head with a small or absent physiologic cup, abnormal branching of the central vessels and full nerve fiber bundles that obscure the disc margin.11,21,28 Systemic risk factors of NAION include arterial hypertension and hypotension, atherosclerosis, sleep apnea, migraine and arteriosclerosis.4,11,28,29
The exact pathophysiology of NAION is not fully known, although it is generally accepted that the delayed optic disc filling via branches of the short posterior ciliary arteries in the retolaminar region of the optic nerve ultimately causes nerve cell death. In terms of systemic risk factors, investigators believe nocturnal hypotension to be the final insult for the development of non-arteritic ischemic optic neuropathy in patients with a disc at risk.21
There is currently no consistently effective therapy for NAION, and observation is typically recommended with testing, including: complete ocular examination, visual field, blood work such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) if GCA is suspected and referral to internal medicine for the management of any underlying vascular issues.28
The most common treatment for NAION is oral steroids.28 Researchers found that systemic corticosteroids were effective in improving visual function compared with the natural history; however, the untreated group had more vascular risk factors than the treated group.28 They treated patients with 80mg/day of prednisone with a gradual taper over several weeks, although it is not considered standard of care.28 Intravitreal Avastin (Genentech) was studied as a proposed method of increasing perfusion to the ONH, but investigators found it had no beneficial effect. Other treatments studied include diphenylhydantoin, erythropoietin and hyperbaric oxygen, although none showed a consistent benefit.28
• NAIONs and PDE5 inhibitors. Phosphodiesterase-5 (PDE5) inhibitors are often used in the treatment of erectile dysfunction (ED). PDE5 is a naturally occurring enzyme that works to inhibit smooth muscle relaxation. PDE5 inhibitors promote smooth muscle relaxation, causing vasodilation and facilitating the erectile process.21
Many anecdotal case reports have described NAION in patients who take erectile dysfunction medications, especially PDE5 inhibitors. Researchers hypothesize that these medications have a mild hypotensive effect, which increases physiological nocturnal hypotension and thus results in ONH ischemia in susceptible patients.29 However, a large pharmaco-epidemiological nested case-control study of 1,109 cases of NAION recently found no association between PDE5 inhibitor medication use and a diagnosis of NAION.30
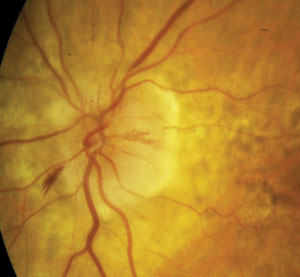 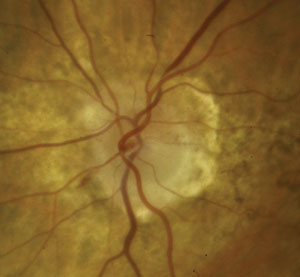 |
| NAION in an 80-year-old white female. At left, the optic nerve on presentation. Note disc edema and hemorrhaging. At right, one month later. Note improved edema and some early inferotemporal disc pallor. Photos: Michael Trottini, OD. |
PDE6, which is found in the retina and is responsible for retina phototransduction, can be inadvertently inhibited by ED medications. This, in turn, can lead to cyanopsia—a bluing of vision—which occurs shortly after taking the medication. The effect of this is transient and is not considered harmful.31
Hypertension
High blood pressure is a well-known cause of adverse ocular sequelae.32-34 Generally, a patient is considered hypertensive when their pressure is 140/90 or higher.35 Hypertension is generally an asymptomatic condition except in cases of malignant hypertension (BP greater than 180/120). Symptoms of malignant hypertension can include headache, nausea, vomiting, nosebleeds and changes in vision.35 Optometrists can identify ocular signs of hypertension and can communicate these findings with the patient's internist or cardiologist for treatment modification.
The recommended first-line treatments of hypertension are: a thiazide-type diuretic (i.e., hydrochlorothiazide), a calcium channel blocker (i.e., amlodipine), angiotensin converting enzyme inhibiors (ACEIs) such as lisinopril or an angiotensin receptor blocker such as Cozaar (losartan, Merck).35 Beta blockers have fallen out of favor as first-line therapy because of the side effects' impact on cardiovascular health, though they remain widely used in patients with heart failure.35-37
• Hypertensive effects on retinal vasculature. When blood pressure is chronically or acutely elevated, the retinal vessels show characteristic changes. Initial response to elevated blood pressure is vasospasm and increase in vasomotor tone, which manifests into retinal-arteriolar narrowing.38 Chronic changes cause focal areas of narrowing (silver wiring) and compression of retinal veins by arteries (arteriovenous nicking). When elevated blood pressure is severe, there can be blood and lipid leakage, as well as focal areas of ischemia (cotton-wool spots) and papilledema. These signs can be predictive of cardiovascular events such as stroke, congestive heart failure and coronary artery disease.34,39-41
When these retinal signs are clinically apparent, it is important to target the microvasculature to reduce risk of morbidity and mortality. This includes tight control of blood sugar in patients with Type 2 diabetes.42 Some evidence suggests that ACEIs and other antihypertensive medications could directly benefit microvasculature in addition to lowering blood pressure.43,44 If retinal emboli are present, anti-coagulation therapy, such as aspirin or Coumadin (warfarin, Bristol-Myers-Squibb) should be a consideration. This should be a coordinated effort between the eye care professional and the patient's cardiologist or primary care doctor.
• Ocular perfusion pressure and hypertension. OPP is very sensitive to changes in blood pressure, especially when ocular autoregulation is impaired.6,45 Changes in the perfusion pressure to the optic nerve in particular have severe consequences for optic nerve health.6,45
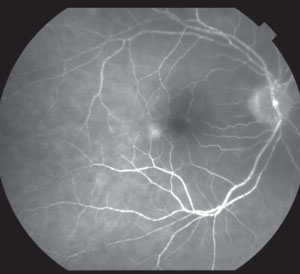 |
| Fluorescein angiogram of a CRAO in a 78-year-old Caucasian woman with a history of bacterial endocarditis. |
Given the formula used to determine ocular perfusion pressure (OPP = arterial BP - IOP), elevated arterial blood pressure should increase ocular perfusion pressure. Although it seems hypertension could have a positive effect on the perfusion of the optic nerve, it is not that simple. Elevated BP leads to short-term constriction of blood vessel walls as the body attempts to control the flow. Long-term, it leads to arteriosclerosis, which will reduce ocular blood flow. Chronic hypertension is also a disease where vascular autoregulation is impaired.6,45 Hypertension has a negative effect on ocular perfusion, contributing to potential glaucomatous damage.6,45
• Retinal vein occlusions and PVD. One of the most common retinal vascular diseases, retinal vein occlusion (RVO) can cause vision loss, dilated tortuous retinal veins, retinal hemorrhages, cotton-wool spots and macular edema.46 Reduced ocular blood flow, glaucoma and PVD all increase the risk of RVO. Atherosclerosis and hypertension are the main risk factors for a vein occlusion; however, hypercoagulopathies and vasculitis are also associated with retinal vein occlusion, particularly in younger patients.32
Retinal artery occlusion is less common. It is divided into central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO). Symptoms are sudden, painless, unilateral vision loss.47-49
A BRAO may cause a visual field defect with minimal effect on vision, while a CRAO causes catastrophic vision loss.50 Risk factors for branch retinal artery occlusion include: aortic and mitral valve disease, acute myocardial infarction, subacute bacterial endocarditis and prosthetic valves.48,49 PVD is also a risk factor for the development of artery occlusion and is often the etiology in young, healthy patients.
It should be noted retinal artery occlusions, specifically CRAO, are true ocular emergencies. Treatment options for retinal artery occlusions are limited in their efficacy. Restoring ocular circulation using digital massage of the eyeball, anterior chamber paracentesis to rapidly lower intraocular pressure and breathing into a paper bag to induce carbon-dioxide-related vasodilation are potential therapies.51,52 Often it is more important to identify the etiology to prevent further complications.
Patients with artery occlusions should undergo echocardiogram and carotid Doppler testing to try and identify the source of the emboli.53 Giant cell arteritis and other vasculitic disorders should also be considered as underlying etiologies of artery occlusions, especially if a retinal embolus is not identified.48,49 The close correlation between PVD and retinal vascular disease and optic neuropathies means that some medications used to promote cardiovascular health may help.33 Lifestyle changes for patients with PVD include not keeping BMI too low, eating a healthy diet of antioxidant-rich fruits and vegetables and avoiding stressful situations.
Secondary Vascular Dysregulation
SVD occurs as a consequence of other diseases, such as multiple sclerosis, retrobulbar neuritis, rheumatoid arthritis, fibromyalgia and giant cell arteritis.5,33 In inflammatory disease processes, molecules release into the corresponding tissue and the blood stream, changing the molecular concentration in the circulating blood and subsequently affecting remote organs.5,33 Of particular ocular interest is the molecule factor endothelin-1 (ET-1).5,13,33,34
• Endothelin and its ocular effects. Endothelin is a potent vasoconstrictor expressed as three isoforms, ET-1, -2 and -3. ET-1 was found to have a strong vasoconstrictive effect on human ophthalmic circulation.13 This effect is exacerbated in diabetic and hypertensive patients where a dysfunction of these endothelial mechanisms in the peripheral arteries already exists.13 As such, ET-1 is believed to play an important role in the pathophysiology of ophthalmic complications by weakening the blood/retinal barrier, leading to retinal edema, hemorrhages and exudate.5,13 Interestingly, patients with retinal vein occlusion have increased plasma levels of ET-1 during the acute phase. Weeks or months later, the concentration decreases, but rarely normalizes completely.5,54
In patients with glaucoma, ET-1 is thought to contribute to endothelial cell dysfunction and may represent the underlying cause of, or at least contribute to, alterations in ophthalmic blood flow.13 ET-1 levels were found to be higher in those with progressive ocular nerve head damage than in those in which the damage had stabilized.13 Increased plasma ET-1 levels have been described in normal tension glaucoma patients, although this finding was not confirmed in multiple studies.13 Using animal models, researchers found chronic administration of ET-1 can produce an optic neuropathy similar to glaucoma.13
ET-1 is a potential target for future pharmacological intervention that may provide treatment for ocular diseases for which no effective drug therapy is currently available.13 ET blockers have been proposed and preliminarily studied to reduce vasospasm; however, routine use is not yet recommended due to potential side effects.5
Our understanding of ocular pathology continues to grow, and eye care providers should always look at the eye as an extension of the cardiovascular system. Learning more about how vascular dysregulation affects the eye will alter our treatment approach to various ocular diseases. Incorporating a multidisciplinary approach will allow for better treatment of not only the eye, but for the body as whole.
Dr. Tolud is in practice at South Jersey Eye Physicians, in Columbus, NJ.
Dr. Harewood is in practice at Staten Island University Hospital, Retina Center in Staten Island, NY.
References
- Flammer J, Haefliger IO, Orgul S, Resink T. Vascular dysregulation: a principle risk factor for glaucomatous damage? J Glaucoma. 1999;8:2121-19.
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms under-lying blood flow and regulation in the retina and choroid in health and disease. Progress Retin Eye Res. 2012;31:377-406.
- Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002 Jul;21(4):359-93.
- Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76-96.
- Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 2013; 4(1):14.
- Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA Journal. 2013;4:14.
- Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol. 2009 Mar;20(2):73-8.
- Perry RB, Rose JC. The clinical measurement of retinal arterial pressure. Circulation. 1953;18:864-70.
- Costa VP, Harris A, Anderson D, et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmologica. 2014:92e252-e255.
- Kaiser JH, Flammer J, Messerli J. Vasospasm – a risk factor for nonarteritic anterior ischemic optic neuropathy? Neuro-ophthalmology. 1996;16(1):5-10.
- Hayreh SS. The Role of age and cardiovascular disease in glaucomatous optic neuropathy. Survey of Ophthalmology. 1999;43(1):27-42.
- Yamamoto T, Kitazawa Y. Vascular pathogenesis of normal-tension glaucoma: possible pathogenic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Progress in Retinal and Eye Research. 1998;17(1);127-43.
- Salvatore S, Vingolo EN. Endothelin-1 role in human eye: a review. J Ophthalmol. 2010;2010:354645
- Ostrow CL. Use of the Trendelenurg position by critical care nurses: Trendelenburg survey. Am J Crit Care. 1997 May;6(3):172-6.
- Krupin T, Liebmann JM, Greenfield DS, et al. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011 Apr;151(4):671-81.
- Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014 Sept;16;9:1563-71.
- Cioffi, G. Three common assumptions about ocular blood flow in glaucoma. Surv of Ophthalmology. 2001;45(3)S325-S331.
- Anderson D. Normal-tension glaucoma (Low-tension glaucoma). Indian J Ophthalmol. 2011 Jan; 59(Suppl1):S97-S101.
- Collaborative Normal-Tension Glaucoma Study Group: The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:495-505.
- Tanna A. Managing NTG: Strategies for success. Review of Ophthal. 2009 Feb;16(2):70.
- Schumer RA, Podos SM. The nerve of glaucoma! Arch. Ophthalmol. 1994;112:37-44.
- Pechere-Bertschi A, Sunaric-Megevand G, Haefliger I, et al. Renal sodium handling in patients with normal pressure glaucoma. Clin Sci (Lond). 2007 Jun;112(6):337-44.
- Shim SH, Kim JM, Choi CY, et al. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J Med Food. 2012 Sep;15(9):818-23.
- Chung HS, Harris A, Kristinsson JK, et al. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15:233–40.
- James CB, Smith SE. The effect of posture on the intraocular pressure and pulsatile ocular blood flow in patients with non-arteritic anterior ischaemic optic neuropathy. Eye. 1991;5:309-14.
- Kim IG, Kim DY. Anterior ischemic optic neuropathy associated with udenafil. Korean J Ophthalmol. 2012;26(3):235-38.
- Burde RM. Optic disk risk factors for non-arteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1993;116:759-64.
- Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye. 2015;29:65-79.
- Hayreh SS. Erectile dysfunction drugs and non-arteritic anterior ischemic optic neuropathy: Is there a cause and effect relationship? Journal of Neuro-Ophthalmology. 2005 Dec;25(4):295-98.
- Nathoo NA, Etminan M, Mikelberg FS. Association between phosphodiesterase-5 inhibitors and nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2015 Mar;35(1):12-5.
- Smith WB. PDE5 inhibitors: considerations for preference and long-term adherence. Int J of Clin Pract. 2013;67(8):768-80.
- Wong T, Mitchell P. The eye in hypertension. Lancet. 2007;369:425-35.
- Flammer J, Konieczka K, Bruno RM, et al. The eye and the heart. European Heart Journal. 2013;34:1270-8.
- Duncan BB, Wong TY, Tyroler HA, et al. Hypertensive retinopahy and incident coronary heart disease in high risk men. BR J Ophthalmol. 2002;86:1002-6.
- James PA, Oparil S, Carter B, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the eighth joint national committee. JAMA. 2014;311(5):507-20.
- Dahlof B, Devereux RB, Kjeldsen SE, et al. LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE); a randomized trial against atenolol. Lancet. 2002;359(9311):995-1003.
- Antihypertensive and Lipid-lowering Treatment to Present Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as a first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2003;42(3):239-46.
- Gugleta K, Orgül S, Stümpfig D, et al. Fludrocortisone in the treatment of systemic hypotension in primary open-angle glaucoma patients. Int Ophthalmol. 1999;23(1):25-30.
- Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ. 1991;302:499-504.
- Michelson EL, Morganroth J, Nichols CW, MacVaugh H 3rd. Retinal arteriolar changes as an indicator of coronary artery disease. Arch Intern Med. 1979;(139):1139-41.
- Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63-9.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-13.
- Levey BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735-40.
- Arnold JM, Yusuf S, Young, et al. HOPE Investigators. Prevention of heart failure in patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:1284-90.
- Lee J, Choi J, Jeong D, et al. Relationship between daytime variation in blood pressure or ocular perfusion pressure in glaucomatous visual field progression. Am J Ophthalmol. 2015:1-15.
- Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli. Ophthalmol. 2005;112:540-7.
- Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493-519.
- Recchia FM, Brown GC. Systemic disorders association with retinal vascular occlusion. Curr Opin Ophthalmol. 2000;11:462-7.
- Wilson LA, Warlow CP, Russel RW. Cardiovascular disease in patients with retinal arterial occlusion. Lancet. 1979;1:292-4.
- Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Current Opinion in Ophthalmology: 2000 Dec;11(6):462-7.
- Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmol. 1995;102:2029-34.
- Rumelt S, Dorenboim Y, Rehany U. Aggressive systemic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128:733-8.
- Wong TY, Klein R. Retinal arteriolar amboli: epidemiology and risk of stroke. Curr Opin Ophthalmol. 2002;13:142-6.
- Haufschild T, Prünte C, Messerli J, Flammer J. Increased endothelin-1 plasma level in young adults with retinal vascular occlusive diseases. Klin Monbl Augenheilkd. 2004 May;221(5):357-9..
