Know Your Retinal Breaks, Tears and Holes
Learn to better recognize posterior segment damage and minimize patient risk.
Goal Statement:
Prompt diagnosis and proper comanagement of retinal tears, breaks and holes are a necessity. This article discusses the classifications of the different types of retinal tears, breaks and holes, the propensity towards the development of RD, signs and symptoms and risk factors.
Faculty/Editorial Board:
Diana Shechtman, OD, and Marlon Demeritt, OD
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID is 45707-PS. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Drs. Shechtman and Demeritt have no relevant financial relationships to disclose.
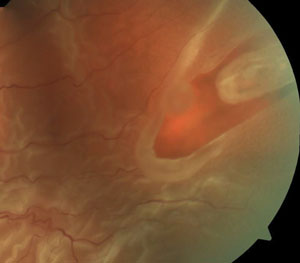
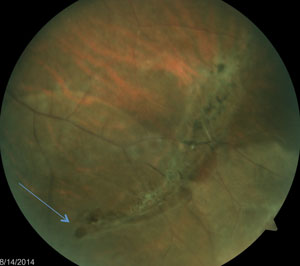 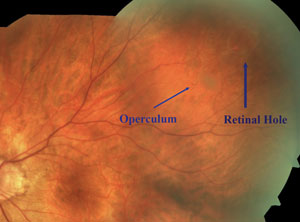  Tears are often found at the edge of lattice degeneration where the retina is thinner (top); the operculum appears as a whitish, disc-shaped floater over the retinal break (middle); atrophic holes are commonly found in the peripheral retina and within lattice retinal degeneration (bottom). |
Retinal tears, breaks and holes are commonly encountered in clinical practice. As they predispose a patient to a retinal detachment (RD) and optometrists are often the first clinician to examine such patients, prompt diagnosis and proper comanagement is a necessity.
This article will review the overall clinical picture of retinal holes, tears and breaks. It will discuss the classifications of the different types—specifically, horseshoe (flap) tears, operculated retinal breaks and atrophic holes—the propensity towards the development of RD, signs and symptoms and risk factors.
Posterior Vitreous Detachment Basics
Retinal tears, breaks and holes are often complications of posterior vitreous detachment (PVD). Understanding PVD, as well as recognizing its symptoms, can lead to early detection of retinal damage.
The vitreous humor is a transparent gel composed mostly of water. Other components include collagen fibrils and glycosaminoglycans (GAGs) or mucopolysaccharides (primarily hyaluronic acid). Collagen fibrils are structural proteins that add support and shape to the vitreous. They, along with extracellular matrix protein, help enhance vitreoretinal adhesion. In addition, hyaluronic acid provides stability to the collagen fibrils and is associated with the viscoelastic properties of the vitreous gel.
With age, the dynamics of collagen fibrils and hyaluronic acid break down, resulting in liquefaction, shrinkage and collapsing of the vitreous. Sequentially, small holes develop within the posterior vitreal cortex, causing liquefied vitreous to gain access to the subhyaloid space. This, along with a weakening of the vitreoretinal adhesion, leads to a separation of the vitreous from the retina’s internal limiting membrane, resulting in PVD.
Sixty-five percent of people over the age of 65 will experience a PVD.1,2 The condition typically occurs between the ages of 45 and 65 in the general population; however, the posterior vitreous may detach earlier in patients with retinal vascular diseases, trauma, aphakia, inflammation, vitreous hemorrhage or myopia.3-7 A characteristic PVD is described as a ring shape overlying the optic nerve, referred to as a Weiss ring.
Up to 26% of patients with an acute PVD will present with a concomitant retinal break at the time of the initial presentation.8-12 The chances of developing a retinal break following the initial presentation of an acute PVD is 2% to 5%.10,13,14
PVD Symptoms
Symptoms associated with a posterior vitreous detachment include flashes or floaters, or both. Patients typically report the characteristic flashes (photopsias) as an arc-shaped light perceived in their peripheral vision, which is most noticeable in the dark. Such photopsias are likely the result of vitreous traction on the peripheral retina, stimulating the underlying photoreceptors. In addition, it is also possible that the typically encountered arc-shaped flash is due to strong adhesion of the vitreous to the margin of the optic nerve head. As traction develops on this adhesion site, all the fibers that enter the optic nerve in that area become mechanically stimulated, resulting in an arc-shaped flash.
Flashes seem to represent a more ominous symptom than floaters. Though floaters are commonly associated with PVDs, they may be linked to other vitreal abnormalities such as blood or condensation of vitreous collagen. Floaters simply represent the casting of shadows onto the underlying retina.
Anomalous PVD
Complications of PVD are more likely to occur in eyes when accelerated vitreous liquefaction occurs prior to the weakening of the vitreoretinal adhesion, a phenomenon known as an anomalous PVD. This entity leads to traction at the vitreoretinal interface due to incomplete separation. Predisposed areas of strong vitreoretinal adhesions include the vitreous base, edge of lattice degeneration, blood vessels, optic nerve, macula and chorioretinal scars. Anomalous PVD can lead to numerous complications affecting the peripheral retina and the posterior pole (see Table 1).15
Peripheral Retinal Breaks
There are three common classifications of retinal breaks to look out for when evaluating patients:
An operculated retinal tear occurs following significant vitreous traction in a small, discrete area of the retina, resulting in increased vitreo-retinal adhesion with an avulsed piece of retinal tissue (an operculum) overlying a small hole. Operculated holes are described as a plug of sensory retina overlying a round retinal break. It is often asymptomatic and results from a PVD pulling onto a vitreoretinal tuft.17
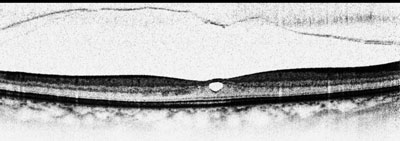 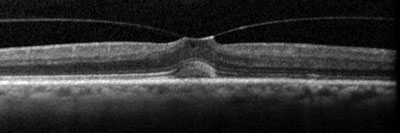 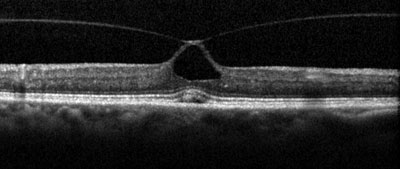 OCT shows a complete PVD overlying a small macular defect (top); examples of anomalous PVD, represented by vitreomacular traction (middle and bottom). |
The operculum, which is seen as a whitish, disc-shaped floater over the retinal break, moves with eye movements due to its attachment to the posterior vitreous cortex. The operculum often shrinks with time, becoming smaller than the overlying break, indicating chronicity.17 As a result, larger opercula are likely more acute in nature.
Operculated tears are usually round and appear redder than the surrounding retina since they are a full-thickness retinal defect. Although they can occur in any region of the retina, they are generally located between the ora serrata and the equator.17 Operculated breaks usually do not progress to rhegmatogenous retinal detachment (RRD) because of release of the vitreous traction to the involved area during the formation of the operculum.17 Traction on an incomplete operculated hole may increase the propensity to develop a retinal detachment. This presentation is often associated with symptomatic operculated breaks.18,19 Thus, determining the presence of traction or symptoms of flashes and floaters in such cases becomes important in guiding treatment.
When significant localized vitreous traction exists, a horseshoe (flap) tear can develop. This is also known as a U-shaped tear or incomplete break. Horseshoe tears occur as a result of incomplete vitreous traction pulling a horseshoe-shaped thin curvilinear flap of sensory retina towards the vitreous cavity.
| Case Study: Horseshoe Tear Follows Acute PVD |
A 60-year-old white male presented with two-day onset of complaints of flashes and floaters affecting the right eye. He reported that the symptoms occurred suddenly. His ocular history was remarkable for trauma in the left eye many years ago, but his medical history was unremarkable. Upon examination, his uncorrected visual acuity measured 20/50 (improving with pinhole to 20/30) OD and 20/25 OS. All preliminaries, including confrontation visual fields, extraocular motilities and pupillary testing, were unremarkable. Examination of his anterior segment was also unremarkable in both eyes. Both eyes' IOP measured 12mm Hg. A dilated fundus exam of the vitreous revealed only a posterior vitreous detachment in the right eye, in addition to trace syneresis in the left eye. We saw no evidence of hemorrhage or pigmented cells in the vitreous of either eye. Optic disc cupping was 0.3/0.3 with pink, distinct neuroretinal rims in both eyes. Evaluation of both maculae revealed normal uniform color with no associated thickness or abnormalities. The peripheral retina was evaluated with scleral depression. No holes, tears or detachments were visible in either eye. We made the diagnosis of acute PVD in the right eye, advised the patient of the findings and educated him on new signs and symptoms that might occur. If they did, the patient was asked to return to the clinic immediately; otherwise, we recommended the patient return for a follow up in a week. The patient returned in a week as advised. His case history revealed stability of the flashes and floaters with no changes in his signs or symptoms. His uncorrected visual acuities and preliminary testing remained unchanged from the previous visit. His anterior segment was unremarkable. IOP was 14mm Hg OD and 15mm Hg OS. Dilated fundus exam revealed vitreal pigmented cells and a Weiss ring in the right eye. Trace vitreal syneresis was noted in both eyes. Peripheral findings of the left remained unremarkable, however a shallow rhegmatogenous retinal detachment associated horseshoe tear was observed in the temporal retina of his right eye with no macular involvement noted. We immediately referred this patient to a retina specialist for treatment. |
The apex of the flap tear usually points toward the posterior pole, while the base points to the anterior retina. Although flap tears can exist in any region of the peripheral retina, they are most often found near the posterior margin of the vitreous base and at the edge of lattice degeneration, because the retina is thinner in the periphery and in areas with lattice degeneration.17 Although lattice retinal degeneration has a very low risk of developing into RRD, patients with lattice and tractional-related flap tears associated with PVD have significantly increased risk of developing a rhegmatogenous retinal detachment.20,21
Flap tears are the most ominous type of tears due to their persistent traction on the retina. Approximately 50% of those with symptomatic flap tears will develop an RRD.20,22,23 If retinal detachment is to follow, it usually does so within a few weeks after tear formation.18 Horseshoe tears discovered in asymptomatic fellow eyes are less likely than symptomatic ones to develop an RRD. Thus, symptoms may be an important element in determining treatment of such tears. Patients presenting with acute retinal breaks or symptomatic retinal breaks should be referred to a retinal specialists for evaluation and management.
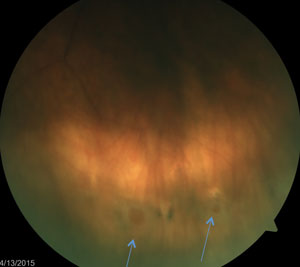
By far the most common presentation of retinal tears is atrophic holes. Although they are not a result of tractional forces, they can present as a risk factor for developing a rhegmatogenous retinal detachment. An atrophic retinal hole is described as a full-thickness retinal break, commonly round-shaped with or without surrounding pigment. Associated pigment is a sign of chronicity and carries a very low risk of developing an RRD. Atrophic holes are a result of an atrophic process associated with a thinning retina or vascular insufficiency. They are more commonly found in the peripheral retina and within lattice retinal degeneration. Holes within lattice retinal degeneration may increase the propensity of progressing towards an RRD, although the risk is still quite small. The sizes of these holes vary from pinpoint to greater than a disc diameter.24
While the pathogenesis of atrophic holes isn’t related to vitreo-retinal traction, vitreous fluid could potentially percolate underneath the hole, resulting more likely in a sub clinical RRD. A subclinical RRD is defined as a >1DD extension of fluid surrounding the hole (larger than a cuff of fluid) but does not extend >2DD into the posterior pole. About one-third of subclinical RRD will progress towards a clinical RRD.25
Evaluation
The initial evaluation of a patient with an acute PVD includes a careful case history. Since symptoms may carry a greater propensity towards the development of an RRD, determining associated symptoms related to flashes and floaters is critical.
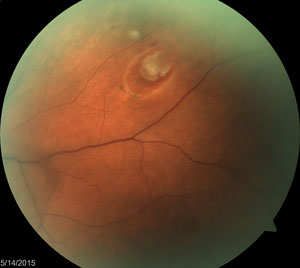 Patient with recent onset PVD with secondary vitreous hemorrhage and horseshoe tear seen clearly, as well as operculated retinal hole seen in the upper left corner of the image. |
|
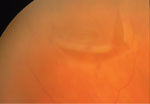 |
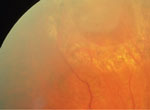 |
| The apex of a horsehoe tear usually points toward the posterior pole and the base points to the anterior retina (left). Patients with horseshoe tears associated with PVD have significantly increased risk of developing an RRD unless treated by laser prophylaxis, as seen here (right). | |
Because PVDs associated with flashers carry a higher risk of developing a concomitant retinal break, it is prudent to reevaluate any increase in signs or symptoms during the follow up of a PVD.26
During the course of the examination, pay careful attention to the vitreous. Evaluate not only for the presence of PVD, but any hemorrhages or pigmented cells within the vitreous. Fifty percent to 70% of vitreous hemorrhages associated with a PVD are also associated with a concomitant retinal break. Retinal breaks or RRD are often associated with pigmented cells in the vitreous. These cells represent pigment liberated from the underlying RPE due to tractional forces.
Since retinal breaks are often found in the peripheral retina, evaluation of the peripheral retina should include careful dilated assessment with indirect ophthalmoscopy. Scleral depression and 3-mirror contact fundus lens can also be helpful in the evaluation of the peripheral retina.
Various auxiliary tests may be useful when evaluating a patient presenting with an acute PVD.
In addition, OCT can be used to aid the clinician in evaluating the stage of the PVD, as well as any associated maculopathy. While a complete PVD may be noted using DFE or OCT, it does not preclude the possibility that associated peripheral vitreoretinal disease may coexist. B-scan ultrasonography has also been found valuable when attempting to assess a fundus that is obscured due to media opacities or vitreous hemorrhage.
Management
Referral for treatment of a retinal break varies based on type of break, associated findings (see Table 2), symptomology and risk factors (see Table 3). Because most atrophic and operculated holes do not have a high propensity towards progression to an RRD, most are followed. On the other hand, if patients display increased risk factors, such as a pseudophakic patient with a posterior capsular rupture, there may be a higher concern for the progression to an RRD. Since 30% of symptomatic retinal breaks progress towards developing an RRD, they should be referred to a retina subspecialist. Remember that symptomatic flap tears carry the highest risk of developing an RRD. See Table 4 for a comprehensive review of the AAO guidelines for treating retinal breaks.
The goal of treating a retinal break is to create a firm chorioretinal adhesion on the attached retina adjacent to and surrounding the retinal tear. To achieve this, the surgeon uses cryotherapy or argon laser photocoagulation to halt the progression of subretinal fluid from detaching uninvolved neurosensory retina.25
Cryotherapy is a method of freezing the retinal break area with the application of a cryo probe to the retinal breaks. The areas of concern are frozen until they are sealed by a chorioretinal adhesion.
Argon laser photocoagulation is most commonly employed and is considered a safe and effective treatment for symptomatic flap tears and other high-risk retinal breaks. Laser burns are applied in several rows around the retinal break to help achieve chorioretinal adhesion.
Regarding time for chorioretinal adhesion, cryotherapy produces a protective barrier in seven to 10 days, and laser photocoagulation achieves its barrier in two to three days. However, cryotherapy is often reserved for cases that cannot be treated with laser. Such cases may include those with difficulty viewing the retina due to opaque media or small pupils. Additionally, using argon laser photocoagulation may be limited by the size of the retinal break and the peripheral location.27
Treatment of peripheral horseshoe tears should be extended to the ora serrata. If the tear is not adequately treated, continued vitreous traction may extend the tear beyond the treated area and allow fluid to dissect through the subretinal space, causing a clinical RD.28-30
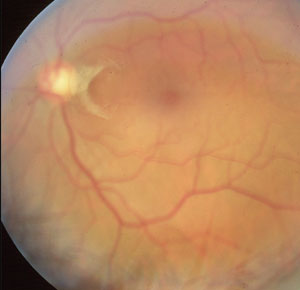 A characteristic PVD such as this one is described as a ring shape overlying the optic nerve, referred to as a Weiss ring. |
When reevaluating a patient following treatment of the tear, it is important to note the presence of a well-sealed barrier, in addition to the absence of new breaks anywhere in the retina. During follow-up visits, it is important for the clinician to evaluate for complications such as an epiretinal membrane (ERM) or macular pucker. Proliferation of the ERM or macular pucker has been observed following treatment for a retinal break. However, although the development of an ERM or macular pucker is possible, research has not shown a direct cause-and-effect relationship.26 In one long-term followup study, the percentage of eyes that developed macular pucker after treatment of retinal breaks was no greater than that of eyes observed to have macular pucker before undergoing treatment.28
Dr. Demeritt is an assistant professor and attending optometrist at Nova Southeastern University College of Optometry and The Eye Care Institute.
Dr. Shechtman is a professor of optometry at Nova Southeastern University College of Optometry, and an attending optometric physician at the Eye Institute and Diabetic/Macula Clinic.
| Principles of PVD Management |
As primary care providers, optometrists are usually the first to evaluate patients with acute posterior vitreous detachments, associated retinal breaks, or both. Since any acute PVD may lead to a rhegmatogenous retinal detachment or retinal break, all acute PVDs must be carefully evaluated and followed. According to the American Academy of Ophthalmology guidelines published in 2014, follow up on an acute PVD should occur anywhere from one week to six weeks following the onset of symptoms, depending on risk factors, associated signs or symptoms, or both.1 Similarly, according to guidelines published by the American Optometric Association, a patient with a symptomatic posterior vitreous detatchment should be followed at least every two to three weeks until the condition becomes asymptomatic and no concomitant retinal tears are found.2 Patients with acute PVDs should be advised to return immediately if they experience an increase in signs or symptoms such as flashes, floaters or a curtain in their vision. More than 80% of retinal detachments are associated with a retinal break that developed from a concomitant PVD.3,4 Since 26% of patients with an acute posterior vitreous detachment will present with a concomitant retinal break at the time of the initial presentation, it may be best to follow all PVDs sooner rather than later.5 1. Brod RD, Flynn HW Jr, Lightman DA. Asymptomatic
rhegmatogenous retinal detachments. Arch Ophthalmol.
1995;113:1030-2. |
References
- Foos RY. PVD. Trans Am Acad Ophthalmol Otolaryngoal. 1972;76:480-97.
- Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreoretinal disease. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:690-8.
- Johnson MW. Posterior vitreous detachment: evolution and role in macular disease. Retina. 2012;32 Suppl2:S174-8.
- Farve M, Goldmann H, Zur Genese der hinteren Glaskorperabhebung. Ophthalmologica. 1956;132:87-97.
- Maumenee IH. Vitreoretinal degeneration as a sign of generalized connective tissue diseases. Am J Ophthalmol. 1979;88:432-49.
- Hikichi T, Trempe CL. Ocular condition associated with posterior vitreous detachment in young patients. Ophthalmic Surg Lasers. 1996;27:782-6.
- Snead MP, Snead DR, James S, Richards AJ. Clinicopathological changes at the vitreoretinal junction: posterior vitreous detachment. Eye (Lond). 2008;22:1257-62.
- Tasman WS. Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthamol Otolaryngol. 1968;72:217-24.
- Tani P, Robertson DM, Langworthy A. Rhegmatogenous retinal detachment without macular involvement treated with scleral buckling. Am J Ophthalmol. 1980;90:503-8.
- Coffee RE, Westfall AC, Davis GH, et al. Symptomatic posterior vitreous detachment and the incidence of delayed retinal breaks: case series and meta-analysis. Am J Ophthalmol. 2007;144:409-13.
- Benson WE, Grand MG, Okun E. Aphakic retinal detachment: management of the fellow eye. Arch Ophthalmol. 1975;93:245-9.
- Scott IU, Smiddy WE, Merikansky A, Feuer W. Vitreoretinal surgery outcomes: impact on bilateral visual function. Ophthalmology. 1997;104:1041-8.
- Dayan MR, Jayamanne DG, Andrews RM, Griffiths PG. Flashes and floaters as predictors of vitreoretinal pathology: is followup necessary for posterior vitreous detachment? Eye (Lond). 1996;10:456-8.
- van Overdam KA, Bettink-Remeijer MW, Mulder PG, van Meurs JC. Symptoms predictive for the later development of retinal breaks. Arch Ophthalmol. 2001;119:1483-6.
- Johnson MW. Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537-67.
- Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371-82.
- Jones WL, Cavallerana AA, Morgan KM, et al. Care of the patient with retinal detachment and related peripheral vitreoretinal disease. American Optometric Association. 1999:1-73.
- Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92:183-94.
- Colyear BH Jr, Pischel D. Preventive treatment of retinal detachment by means of light coagulation. Trans Pac Coast Oto-Ophthamol Soc. 1960;41:193-217.
- Byer NE. Rethinking prophylactic therapy of retinal detachment. In: Stirpe M, ed. Advances in Vitreoretinal Surgery. New York, NY: Ophthalmic Communications Society; 1992:399-411.
- Byer NE. Long-term natural history of lattice degeneration of the retina. Ophthalmology. 1989;96:1396-402.
- Byer NE. What happens to untreated asymptomatic retinal breaks, and are they affected by posterior vitreous detachment? Ophthalmology. 1998;105:1045-50.
- Neumann E, Hyams S. Conservative management of retinal breaks: a follow-up study of subsequent retinal detachment. Br J Ophthalmol. 1972;56:482-6.
- Schepens CL. Retinal detachment and allied diseases, vol 1. Philadelphia: WB Saunders; 1983:158-60.
- Brod RD, Flynn HW Jr, Lightman DA. Asymptomatic rhegmatogenous retinal detachments. Arch Ophthalmol. 1995;113:1030-2.
- American Academy of Ophthalmology Preferred Practice Patterns Committee. Preferred Practice Pattern Guidelines. Comprehensive Adult Medical Eye Evaluation. San Francisco, CA: American Academy of Ophthalmology; 2010.
- Pollak A, Oliver M. Argon laser photocoagulation of symptomatic flap tears and retinal breaks of fellow eyes. Br J Ophthalmol. 1981;65:469-72.
- Robertson DM, Norton EW. Long-term follow-up of treated retinal breaks. Am J Ophthalmol. 1973;75:395-404.
- Benson WE, Morse PH, Nantawan P. Late complications following cryotherapy of lattice degeneration. Am J Ophthalmol. 1977;84:514-6.
- Delaney WV Jr. Retinal tear extension through the cryosurgical scar. Br J Ophthalmol. 1971;55:205-9.