CXL: A First-line Therapy for Keratoconus
Eye care professionals have a new leading treatment option to combat this disease.
By Clark Y. Chang, OD, MSA, MSc, and Christopher J. Rapuano, MD
Release Date:
April 27, 2018
Expiration Date:
April 27, 2021
Goal Statement:
Prevalence rates for keratoconus could be as high as one in 375 in general poplations, according to recent research. As such, management of this disease is more important than ever. This article provides a comprehensive review of corneal collagen crosslinking, which could provide clinicians with a safe and effective first-line therapy for keratoconus patients.
Faculty/Editorial Board:
Clark Y. Chang, OD, MSA, MSc, and Christopher J. Rapuano, MD
Credit Statement:
This course is COPE approved for 1 hour of continuing education credit. Course ID is 57903-AS. Check with your state licensing board to see if this counts toward your CE requirements for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Authors: Dr. Chang has a speaker's bureau arrangement with Avedro.
Dr. Rapuano is a consultant for Allergan, Avedro, Bio-Tissue, GSK, Novartis, Shire, Sun Ophthalmics and TearLab. He is also a stock shareholder for RPS and has speaker's bureau arrangements with Avedro, Bio-Tissue and Shire.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian and Michael Iannucci all have no relationships to disclose.
 |
| Fig 1. Riboflavin staining in aqueous seen here after 30 minutes of riboflavin loading during CXL treatment. Click image to enlarge. |
Under the conventional keratoconus (KCN) management model, clinicians have generally accepted the notion that an earlier KCN diagnosis is inconsequential, as early detection will not alter the disease's natural course or prevent onset of visual deficits. This gave rise to a simple binary treatment plan for all KCN patients: spectacles or contact lenses for those with early to moderate disease, and corneal transplantations for the 10% to 20% who either have severe KCN or cannot tolerate contact lens wear.1,2
Notwithstanding, even a well-performed keratoplasty has risks such as donor tissue rejection, infection, cataract, glaucoma, ocular surface disease, anisometropia and irregular corneal astigmatism.3 Thus, clinicians generally reserve keratoplasty as a last resort.
But today's access to corneal collagen crosslinking (CXL) shatters this binary treatment path and opens the door to a whole new mindset and therapy regimen.
The Old Way of Thinking
The primary goals of KCN management are to restore visual function, preserve quality of life and defer corneal transplantation. Thus, minimizing risk factors that increase a patient's likelihood of needing a corneal transplant is key. One study of 131 KCN eyes reported younger age (≤30), recent diagnosis (≤5 years from time to diagnosis), poor best-corrected visual acuity (BCVA) (20/40 or worse) and greater ectatic steepening (55D or higher) as main risk indicators associated with a higher likelihood of requiring corneal transplantations.1
|
Disease Basics As KCN progresses or when both eyes are affected, the optical consequences such as irregular astigmatism and increased higher-order aberrations escalate. Here, patients will start to experience wide-ranging visual compromises leading to loss of BCVA. The onset of KCN is often during puberty but can occur even earlier in life, and it tends to progress until around the fourth decade of life, after which relative stability is often observed. Nonetheless, a risk of progression still exists later in life or after a period of stability.1 Clinicians often educate patients that KCN's prevalence rate is approximately one in 2,000.30,31 However, recent epidemiological evidence shows that the KCN prevalence rate may actually be as high as one in 375 among general populations or even higher in certain subgroups.32,33 A recent study of 1,044 pediatric eyes from Saudi Arabia reported a potential pediatric KCN prevalence rate as high as one in 21.33 As such, it's likely the impact of KCN has been grossly underestimated and the clinical consequences underappreciated. |
Since clinicians cannot currently modify the age of onset and have only recently gained the ability to arrest progressive corneal steepening, they traditionally opt to improve BCVA with contact lenses. A well-fitting contact lens can improve visual functions and defer the need for keratoplasty; however, it does not prevent worsening of KCN or further loss of uncorrected visual acuity (UCVA).2,4 In addition, despite use of gas permeable lenses, residual higher-order aberrations and persistent BCVA reductions can still remain.5 The lack of emphasis on KCN stabilization in conventional management could explain why some patients continue to report significant decline in vision-related quality of life.6
Along Came CXL
When first exploring ultraviolet (UV)-induced CXL in the late 1990s, researchers realized the feasibility of a photochemical CXL induction process that produced stiffer corneal tissues with increased resistance to enzymatic digestion and thermal damages.7 Additionally, a landmark pilot study of 23 progressive KCN eyes that underwent CXL reported not only that all eyes were stabilized with no adverse events, but 70% also showed an average reduction of 2.01D in maximal keratometry.8
Needless to say, the advent of CXL instigated a new era of KCN management that now focuses on stabilizing KCN as early as possible. CXL is a photochemical polymerization process in which monomers are rearranged into a three-dimensional network of polymers, subsequently enhancing the tensile strength of a tissue structure.
CXL is also a naturally occurring biological process involving the gradual stiffening of connective tissues over time. The natural, age-associated CXL process is facilitated by lysyl oxidase, an endogenous enzyme derived from the LOX gene pathway (see "Diagnostic Woes," p. 5). It catalyzes the required oxidative reactions to create additional covalent bonds (or "crosslinks") between and within collagen fibrils—yielding increased tissue biomechanical strength and halting KCN progression.9,10
 |
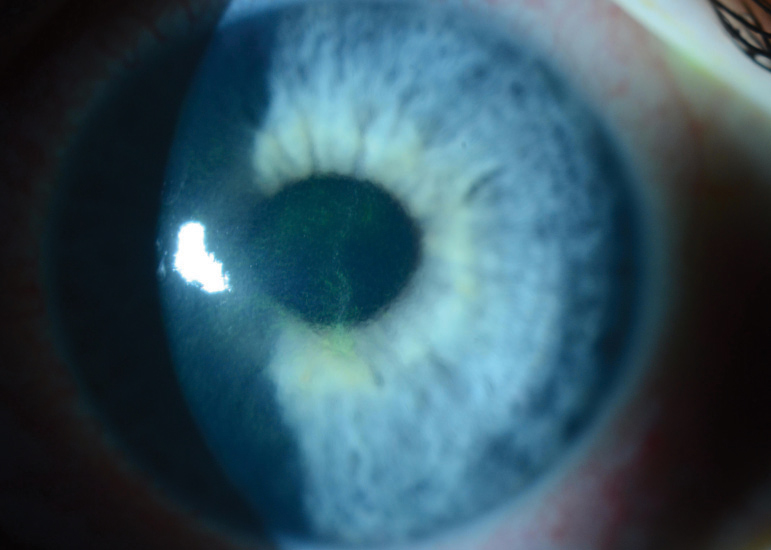
| Fig 2. Advanced keratoconus with Munson's sign. Despite disease severity, note the otherwise clear cornea with no other discernable clinical signs via slit lamp examination. Click image to enlarge. |
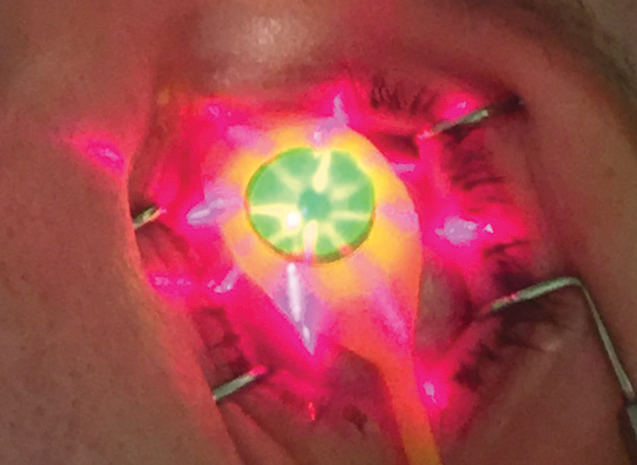 |
| Fig. 3. Crosshair guidance helps clinicians maintain proper working distance between the UV emission device and the treatment site. |
The relative stability observed in many KCN patients after the fourth decade of life is a potential consequence of cumulative age-associated CXL within the corneal stroma. However, the slow and time-dependent natural crosslinking reactions cannot neutralize the cumulative damages accrued in a majority of KCN patients, especially when disease onset has been reported as early as age six.1 Thus, monitoring KCN patients until development of contact lens intolerance while they continue to experience BCVA loss is insufficient to proactively protect patients from the need for a corneal transplantation. Today, accelerating the formation of corneal crosslinks now enables clinicians the possibility of preserving maximal visual functions and halting the rate of ectatic steepening, both of which are protective factors against the need for keratoplasty.1
CXL Dos and Don'ts
To achieve the most efficient and homogenous stromal saturation of photosensitizer molecules within 30 minutes of the riboflavin-loading phase, central epithelium is debrided to fully remove the barriers of epithelial tight junctions. Debridement also disrupts the physiological barrier that slows stromal oxygen replenishment during CXL treatment and removes antioxidant enzymes that can be counter-productive to maximum CXL efficacy. Epithelium shows high levels of antioxidant-acting molecules such as ascorbate and tryptophan residues that can scavenge high-energy reactive oxygen species and impede intended UV transmission from reaching stromal treatment sites.11,12
Standard (epi-off) FDA-approved CXL treatment begins with epithelial removal similarly to a photorefractive keratectomy (PRK). Under topical anesthesia and through standard aseptic technique, the epithelium is removed from the central 9mm. Photrexa Viscous (riboflavin 5'-phosphate in 20% dextran ophthalmic solution, Avedro) is instilled on the cornea in two-minute intervals for 30 minutes.8
Since riboflavin serves as both a photosensitizing agent to propagate CXL chemical reactions and a tissue protector by reducing UV transmittance beyond the intended treatment depth, it is essential that clinicians ensure full saturation within the corneal stroma prior to UV irradiation. Clinicians can achieve this by checking for stromal and aqueous riboflavin staining in the slit lamp after the 30 minutes of riboflavin loading (Figure 1).
A minimum corneal thickness of 400µm, typically measured with an ultrasound pachymeter, is required prior to UV exposure. If pachymetry fails to show proper corneal thickness, hypotonic Photrexa Riboflavin (riboflavin 5'-phosphate ophthalmic solution, Avedro) is administered every five to 10 seconds until the cornea is swollen to at least 400µm.
Minimum corneal thickness of 400µm and full stromal riboflavin saturation help to attenuate UV energy and protect post-stromal structures such as the endothelium, crystalline lens and retina. For example, if a riboflavin-saturated cornea has a minimum pachymetry of 400µm, the calculated UV irradiance will dissipate to about 0.18mW/cm2, which is nearly 50% less than the endothelial damage threshold of 0.35mW/cm2. The energy level projected to reach the lens and retina is even lower and well within their respective damage threshold levels.13,14
 |
| Fig. 4. This patient presented with nearly complete epithelial closure, and the bandage lens remained in position, on day three following CXL treatment. |
Once appropriate riboflavin saturation and pachymetric compliance have been verified, the KXL UV device (Avedro) is programmed for 30 minutes of continuous emission (3mW/cm2) with a calibrated total energy dose of 5.4J/cm2 (Figure 3).8 After initiating UV exposure, Photrexa Viscous continues to be administered in two-minute intervals until treatment conclusion. This maintains maximal concentration of active photosensitizing agents in the stromal treatment zone. The reactive oxygen species are energetically robust and can activate the lysyl oxidase enzymatic pathway. This causes the formation of new covalent bonds within the stromal treatment zone and a stiffer corneal composition.
Post-procedure, clinicians can rinse excess riboflavin off with balanced salt solution. A bandage contact lens should be placed on the treated eye and maintained for three to five days or until epithelial closure (Figure 4). Patients are also typically managed with a topical antibiotic four times a day for one week and a topical corticosteroid four times a day for the first week with a tapering schedule over two to six weeks. The exact prescribed medications, frequencies and length of treatment may vary.15
Future Improvements
Many studies show epi-off CXL renders a good safety profile and high level of efficacy in halting KCN progression.15,16 Some reports also suggest CXL can improve topographic, refractive and aberrometric parameters.8,17,18 Researchers have yet to discover specific patient variables that reliably predict such improvements, so clinicians should continue to educate KCN patients on corneal stabilization as the primary treatment purpose.
|
The Effect of Age If KCN diagnosis cannot be concluded from the baseline presentation, patients should be followed in time intervals that match their risk profile for progression. At Wills Eye Hospital, we believe shorter monitoring intervals of three to six months are justified for patients with one or more of the following characteristics:
Early intervention with CXL can help prevent unnecessary loss of BCVA or UCVA, as well as minimize the need for keratoplasty. |
Although rare, CXL complications exist. Postoperative complications can include the common issues expected with any epithelial delamination process such as variable epithelial healing rate, eye pain, microbial keratitis and sterile infiltrates. Other adverse events associated with CXL include transient corneal haze, persistent corneal edema/endothelial decompensation, corneal scarring, unexplained loss of visual acuity and the need for CXL retreatment due to continued KCN progression (Figure 5).
TE-CXL vs. Epi-off
As safe as epi-off CXL is in clinical practice, some researchers have raised concerns about epithelial removal due to postoperative pain, slow visual recovery and infection risk. The desire to further improve CXL delivery through a minimally disrupted epithelium has fueled numerous clinical studies. While research shows fewer associated adverse events when the epithelium is left mostly intact, some clinical investigations have also demonstrated lower efficacies with transepithelial, or epi-on, CXL (TE-CXL).19-21
TE-CXL patients may be at a higher risk for requiring CXL retreatment. In one study of TE-CXL, pediatric KCN patients showed regression rates of up to 50%. Within a two-year follow-up period, these patients demonstrated continual ectatic steepening after primary TE-CXL, necessitating retreatments with epi-off CXL.17 In addition, a patient noncompliant with follow-up after TE-CXL treatment may have more cumulative risks for continual ectactic steepening. Thus, many researchers still favor the epi-off CXL technique due to its greater treatment efficacy. Nonetheless, because of TE-CXL's quicker recovery course and increased comfort during the initial post-operative period, continual research may bring about enhanced treatment effects in future technologies.
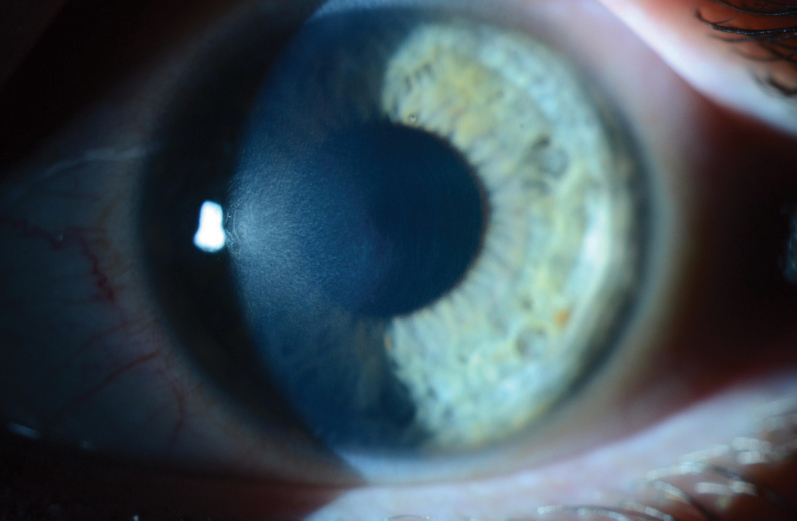 |
| Fig. 5. This patient shows mild CXL-associated haze and demarcation lines seven weeks after CXL treatment. |
Overall, epi-off CXL is considered safe and highly effective, so much so that cornea experts from four international corneal societies recently reached a consensus that clinicians should consider CXL in KCN individuals with high risk profile for progression, even if progression has not yet been documented.22 While what constitutes a high risk profile still needs clarification, it may include young age, severe allergy and eye rubbing.23
How CXL Enhances Management
By using CXL as a first-line KCN treatment, clinicians can induce corneal stability immediately after disease detection and spare patients from experiencing unnecessary hardship with their vision and quality of life. This new management concept can proactively preserve visual function as well as diminish or even eliminate the need for corneal transplantation.24 For example, in countries that adopted the new KCN management paradigm early, CXL is associated with a more than 50% reduction in keratoplasties for KCN patients.25,26
KCN stability after CXL may also enhance success with long-term contact lens wear either by keeping the cornea in a stage that may be easier to fit or by maintaining UCVA and BCVA. Keep in mind that immediately after CXL, epithelial cells may become more fragile and it may take a few months for the initial cellular remodeling to occur. Because persistent epithelial disruption may be associated with corneal opacity development, clinicians need to exercise caution when recommending contact lens refittings after CXL.27 Our clinical guidelines at Wills Eye Hospital are to wait a minimum of four to six weeks after standard epi-off CXL before refitting patients in contact lenses, although the exact time frame will depend on the contact lens type and the CXL procedure itself.28
With the new KCN management approach, clinicians can combine the treatment benefits of CXL and contact lenses to help KCN patients achieve maximal improvement in their lives. Ideally, this combination will significantly reduce the number of patients who will require corneal grafts, a goal once unimaginable with traditional KCN management.
|
Diagnostic Woes KCN patients don't often recognize vision loss until the disease advances in the better-seeing eye; thus, clinicians also cannot rely on patients' subjective visual symptoms to diagnose KCN. Consequently, patients are often left undiagnosed until both eyes are significantly affected. With more severe KCN, visual impairment in at least one eye may no longer be correctable via spectacles or soft contact lenses. |
Dr. Chang is director of cornea specialty lenses at Wills Eye Hospital–Cornea Service and director of clinical services at TLC Vision. Also, he is an advisory board member for the International Keratoconus Academy, the Gas Permeable Lens Institute and the Optometric Cornea, Cataract and Refractive Society.
Dr. Rapuano is chief of Cornea Service at Wills Eye Hospital. He has published several books, numerous book chapters and over 175 peer-reviewed articles, including having co-authored The Wills Eye Manual.
1. Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol. 2005;140(4):607-11. |
