 |
Corneal Pain Presentations: Causes and Interventions
Get up to speed on the basis and manifestations of neuropathic as well as neurotrophic changes.
By Daniel Grangaard, OD, William Hileman, OD, and Steve Njeru, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: April 15, 2024
Expiration Date: April 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists who want to learn about the impact this system has on practice.
Educational Objectives: After completing this activity, participants should be better able to:
Effectively distinguish between corneal pain presentations.
Accurately assess corneal pain in clinical practice.
Recognize the various causes of corneal pain and sensitivity.
Determine the best course of treatment for patients presenting with corneal pain.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Drs. Grangaard, Hileman and Njeru do not have nothing to disclose. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #128217 and course ID 90895-TD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
The cornea is the most powerful pain generator in the body, with an estimated 7,000 nerve terminals per millimeter.1,2 It is 300- to 600-times more sensitive than human skin and provides sensory information that helps relay touch, chemical, pain and temperature signals.1 Its ability to register these signals helps induce tear production, blinking and release of trophic factors to maintain structural integrity.
The nasocillary nerve bundles that innervate the cornea enter the limbus in a radial pattern, then migrate anteriorly to penetrate Bowman’s layer and rest under the basal epithelial layer to form the sub-basal nerve plexus.2,3 These nerves then rise to superficial intra-epithelial terminals, which terminate as bulbous free nerve endings that have nociceptors for sensing pain.2,3 If the corneal nerves are damaged due to injury or disease, the healing process can lead to abnormal regeneration and changes in the regulation of pain receptors. These regulatory changes in the pain receptors may cause either an increase or decrease in pain perception by patients when exposed to stimuli.
 |
|
Fig. 1. The OPAS has a series of questions to gather patient feedback on symptoms related to both ocular pain and corneal pain.4 Click image to enlarge. |
Corneal neuropathic pain, typically a diagnosis of exclusion, describes a heightened perception of pain in the absence of painful stimuli.1 Patients may experience a variety of symptoms such as burning, irritation, dryness, grittiness and most importantly photoallodynia, which is a pain sensation in response to light.1 Neuropathic pain may present without any visible findings during an exam, making it extremely hard to diagnose, especially when patients express severe pain symptoms. This condition may also be referred to as “pain without stain” or phantom cornea.1
By contrast, corneal neurotrophic changes are characterized by a reduction or lack of corneal sensitivity due to impairment of corneal innervation. In this case, the corneal findings are significant and there are little to no symptoms.
Patient Workup
The subjective nature of pain makes clinical assessment challenging, particularly when there is a mismatch between signs and symptoms.
How do you measure corneal pain? Although ocular pain can be a common reason for someone to seek medical attention, it is not always easy for patients to quantify and describe. There are several ophthalmic patient survey instruments available, but most emphasize symptoms of dry eye syndrome rather than ocular pain. So, in 2010, the National Eye Institute workgroup on ocular pain and sensitivity identified a need for a reliable and validated way to assess, measure and quantify ocular pain.4 This led to the creation of the Ocular Pain Assessment Survey (OPAS; Figure 1), which has been validated as a reliable way to quantify not only general ocular pain but also corneal pain.4
The OPAS is a quantitative questionnaire that assesses pain in the past 24 hours and past two weeks by having patients answer questions related to pain intensity, quality of life changes, frequency of pain, aggravating factors, associated factors and symptom relief.4 It is a survey that can be easily administered by both non-clinical and clinical staff as patients complete their treatment plans to determine if their quality of life has improved.
How do you evaluate for corneal pain and sensitivity? Corneal esthesiometry is a tool for measurement of corneal sensation that can be used to detect an underlying neurotrophic component to a patient’s symptoms. These tests may be contact or non-contact and must be performed before using an topical anesthetic.
One of the most common methods is using the Cochet-Bonnet (Figure 2), a handheld device that contains a thin, retractable nylon filament and can extend up to 6cm.5 During this test, it is necessary to document the length when a patient first feels contact, as a shorter length indicates a decrease in corneal sensitivity.5 It can be used to test various discrete regions of the cornea (e.g., central, superior, inferior).
Other test options now include the Corneal Esthesiometer Brill (CEB), which is a recently FDA-approved non-contact method to test corneal sensitivity.5 It can either be used as a handheld device or mounted to the slit lamp and has a camera with two infrared LEDs to ensure a correct testing distance and positioning of the device.5 It works by stimulating the cornea at different pulsed pressures using air at ascending gradients until the patient reports feeling the air.5 This can then be quantified on a grading scale of 1 through 5, with grade 1 indicating corneal hypersensitivity, grades 2 and 3 regular corneal hypersensivity and grades 4 and 5 corneal hyposenstivity.5 This grading scale allows clinicians to monitor for changes in sensitivity in a simple and objective way.
Additionally, clinicians must distinguish between central and peripheral pain, which can be done with topical proparacaine on the cornea. Typically, proparacaine helps relieve peripheral nerve pain without any effect on central pain.1 Therefore, if a patient experiences complete relief with topical 0.5% proparacaine hydrochloride, then they are most likely experiencing only peripheral corneal pain.1 If there is partial relief, then they are most likely experiencing a mixed form of central and peripheral corneal pain.1 However, if there is no relief or the symptoms worsen, then they are most likely experiencing only central corneal pain.1
 |
|
Fig. 2. With a Cochet-Bonnet esthesiometer, you are able to see the markings on the side that reflect the maximum 6cm length of the nylon filament.5 Click image to enlarge. |
Common Causes
There are many conditions that can alter corneal sensory responses and many differential diagnoses to consider.
Dry eye disease. Tear film stability is an essential factor in maintaining corneal integrity. Dry eye is a multifactorial disease characterized by the loss of tear film homeostasis and development of ocular surface inflammation.6 Common symptoms include burning, stinging, blurred vision and a gritty/sandy sensation.
Mild to moderate ocular findings can be subtle and include persistent conjunctival injection, intermittent blurred vision from an unstable tear film, reduced tear break-up time and excessive reflex tearing due to irritation from dryness. In moderate to severe forms of dry eye, persistent surface inflammation can result in corneal abnormalities such as epithelial keratitis, and, in more severe cases, it can lead to persistent epithelial defects and even corneal ulcers from neurotrophic changes.6
A complicating factor is that patients may present with symptoms that do not match the clinical findings as corneal nerve damage occurs from chronic surface inflammation. Chronic dry eye disease has been shown to change corneal sensitivity and lead to a loss in sub-basal corneal nerve density.6 As such, dry eye disease needs to be considered as a contributing factor to both neurotrophic changes and neuropathic pain symptoms.
Recurrent corneal erosion (RCE). This condition causes acute symptoms of pain, tearing, photophobia and blurred vision. The estimated incidence suggests 25.4 cases per 100,000 annually.7 In the acute phase, symptoms are generally secondary to an epithelial defect that can form on any area of the cornea.8 RCEs occur due to a dysfunction of the epithelial cells and the basement membrane complex.
The initial cause of RCE may come from trauma such as corneal abrasion, dystrophies such as epithelial basement membrane dystrophy or even previous corneal infections.8 The painful symptoms experienced by patients are commonly short-lived; however, due to their recurrent nature, patients can repeatedly experience symptoms of pain, burning and blurred vision.8 RCE can have a significant impact on the cornea as it disrupts the healing process, which can cause an upregulation of pain receptors and lead to neuropathic pain.
Surgery. Refractive surgeries—LASIK in particular—are among the most commonly performed surgical procedures in the United States.9 Following LASIK, all patients will experience corneal pain to some extent and there will be potential for a temporary neurotrophic state until the nerve plexus regenerates due to the transection of corneal nerves and loss of superficial innervation following the flap creation. However, some patients can experience symptoms of ocular surface disease as a complication long after the postoperative healing process. Patients with persistent symptoms following LASIK experience pain, spontaneous burning, light sensitivity, sensitivity to air and soreness or aching around the eye.9 These symptoms occur without any significant clinical findings, and patients will often have a limited response to conventional therapies.
The prevalence of neuropathic corneal pain following LASIK is unknown but is estimated to be about one out of 900 patients.9 Many risk factors have been identified, ranging from psychiatric conditions such as depression and anxiety to autoimmune conditions like fibromyalgia and thyroid eye disease.9 A multimodal treatment regimen is recommended for patients with neuropathic pain following LASIK. Treatment options include artificial tears, anti-inflammatory agents, amniotic membranes and, in rare cases, oral pain medication.
 |
|
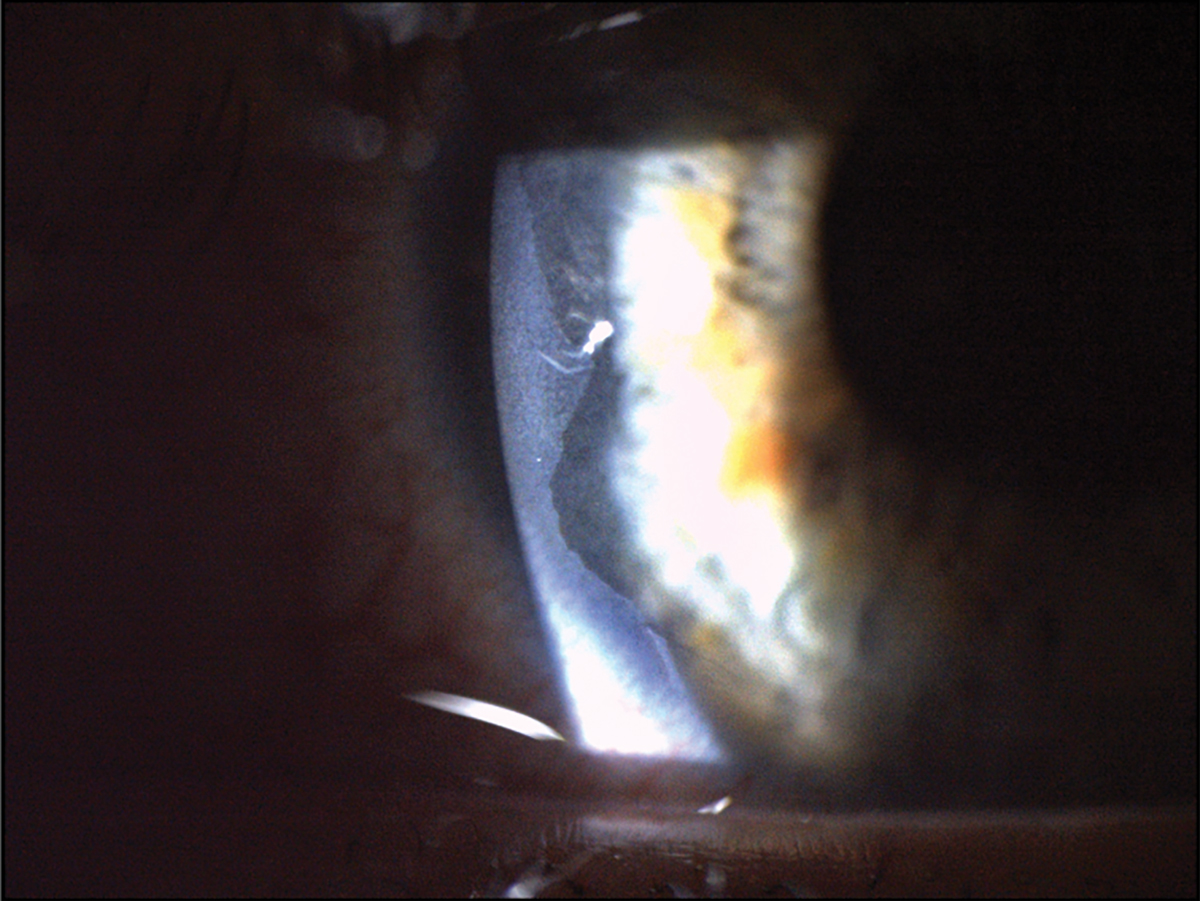
Fig. 3. This patient had a severe chemical burn secondary to exposure from a basic commercial water treatment product. This led to significant epithelial loss that can be seen at the temporal aspect of the cornea near the limbus. Click image to enlarge. |
Chemical burn. Corneal injury resulting in epithelial and corneal nerve density damage is often another etiological factor leading to the development of neuropathic pain. Chemical or thermal burns cause damage to the integrity of corneal innervation and can result in impaired corneal wound healing and prolonged epithelial defects (Figure 3).10
The severity of corneal injury is dependent on the pH of the offending chemical substance. Acidic chemicals denature and coagulate corneal proteins, creating a barrier that prevents penetration into deeper structures.10 Conversely, alkali chemicals are lipophilic and will penetrate deeper structures, causing much more significant ocular trauma.10 Alkali burns are more likely to lead to neuropathic pain, as alkali chemicals have a higher rate of penetration than acids.10 Common symptoms include pain, redness, blurred vision and foreign body sensation.
Patients suffering from a chemical burn require emergent irritation until the ocular surface pH has normalized between 7.0 and 7.2 and will require further treatment with antibiotics, steroids or amniotic membranes.10 Patients who experience chemical or thermal injury should be observed frequently with the existing risk of developing neuropathic pain.
Keratitis. Herpetic eye disease (both simplex and zoster) is a leading cause of neurotrophic keratitis. The herpes simplex virus (HSV) has two subtypes: HSV-1 and HSV-2. The former typically affects the oral, labial and ocular regions.11 HSV-1 will stay latent in the dorsal root ganglion of the trigeminal nerve.11 During reactivation, it travels back to the cornea via the ophthalmic branch of the trigeminal nerve and presents as epithelial, stromal or endothelial keratitis.11 Generally, reactivation can be triggered by fever, hormonal changes, UV exposure, stress, surgeries or trauma.11 The global annual rate of new herpes keratitis cases is about 1.5 million, with an estimated 40,000 new cases of severe visual impairment or blindness each year secondary to HSV-1.11
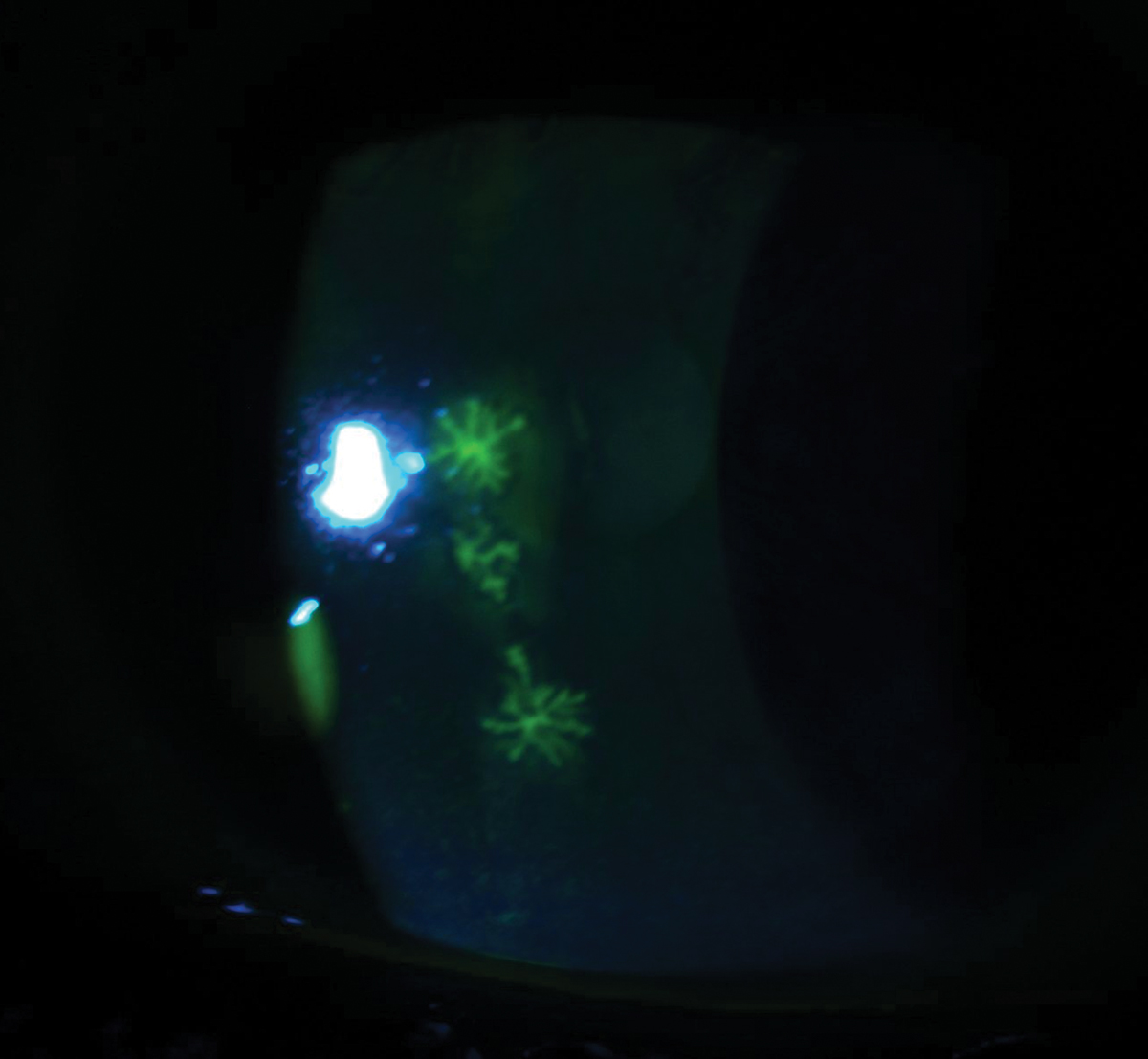
Herpes simplex epithelial keratitis is the most common area of corneal involvement and is responsible for 50% to 80% of ocular herpes infections.12 Within 12 to 24 hours, the infected epithelial cells will form punctate vesicles that are caused by swollen cell nuclei filled with replication DNA viral load, which begins the first stages of a dendrite.13 Next, these infected cells will start to swell, burst and shed the virus, infecting nearby cells and causing the appearance of a dendrite. These dendrites will appear as superficial and branch out in a linear pattern with terminal bulbs that have raised edges (Figure 4).13 Topical dyes such as fluorescein will stain the whole body of the dendrite and improve visibility of the ulcer bed, whereas rose bengal or lissamine green will only stain the terminal bulbs at the margins of the dendrite due to the devitalization of the cells.13
Unfortunately, herpes simplex can affect all layers of the cornea, including the stroma and endothelium during initial infections and/or phases of reactivations due to an inflammatory response from HSV antigens. Herpes simplex can lead to both neurotrophic changes and/or neuropathic pain since it alters corneal sensitivity by either decreasing or increasing sensitivity to stimuli, depending on the severity and/or location of the infection.
 |
|
Fig. 4. This patient was diagnosed with acute herpes simplex keratitis, as these three elevated dendrites were stained with fluorescein. Click image to enlarge. |
The varicella-zoster virus can first manifest as chickenpox during childhood as the primary infection, and then as herpes shingles during a reactivation phase. The primary infection is a result of direct contact with an infected individual who has an itchy vesicular rash across their body.14 Herpes shingles is secondary to the dormant virus that has established latency within the dorsal root ganglion of the nerve; roughly 20% to 30% of infected individuals will have this latency broken, and the virus will present as a painful vesicular rash on the skin that respects the dermatome.14 This reactivation phase is most commonly caused by an immune stress, which can often be seen in advanced aging or illness.14
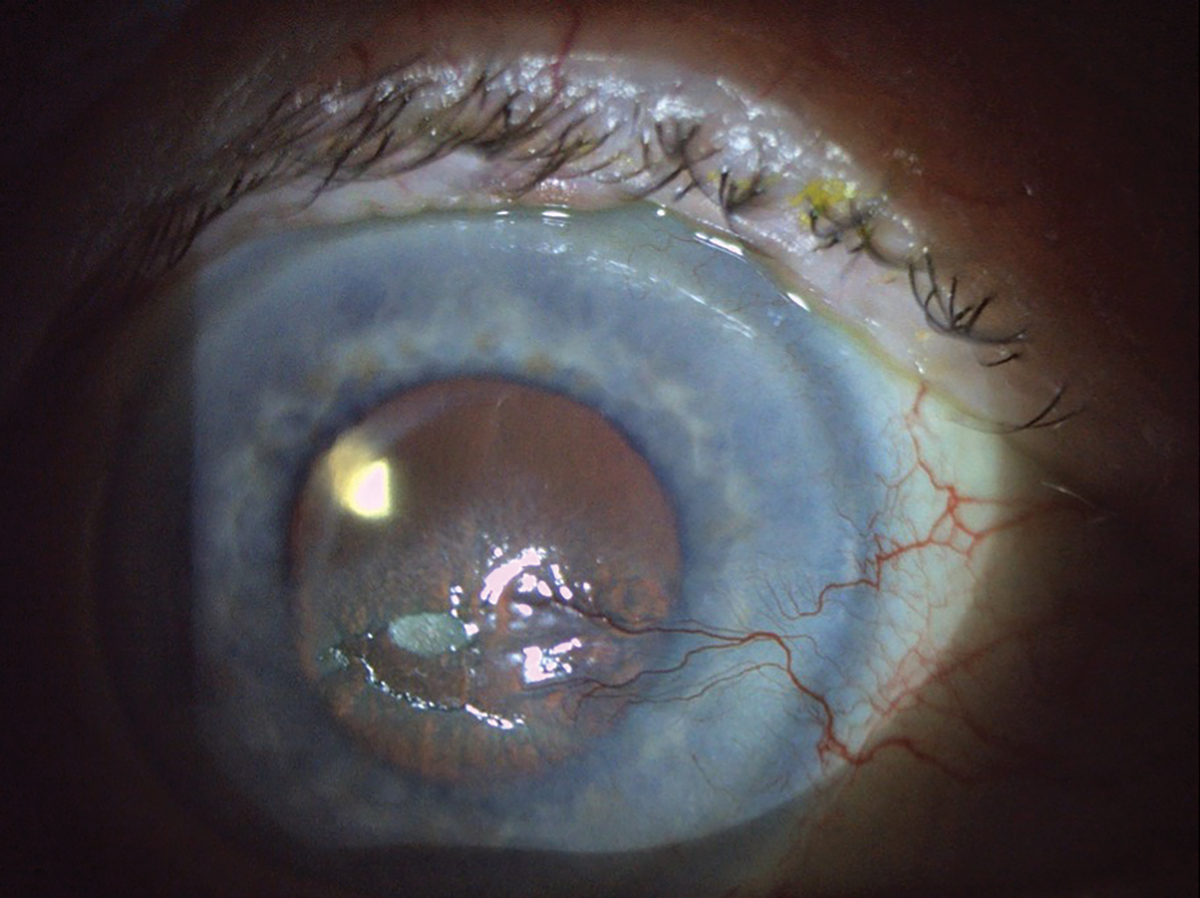
When there is ocular involvement, it is called herpes zoster ophthalmicus, which accounts for roughly 10% to 20% of zoster cases, and can cause significant damage to the cornea (Figure 5).14 The most commonly involved structure is the cornea with herpes zoster keratitis occurring in up to 65% of cases with various manifestations that involve the epithelium, stroma and endothelium.15 Pseudodendrites are a common ocular finding and can appear in 50% to 75% of cases.15 Unlike true dendrites from HSV keratitis, pseudodendrites do not have terminal end bulbs and lack ulceration, so the lesions appear flat.
When treating herpes zoster, optometrists should also be aware of the risk that patients may develop postherpetic neuralgia. This is a neuropathic pain syndrome that can persist or develop even after the rash has resolved with successful treatment. Treating herpes zoster within at least 72 hours will reduce the risk of developing postherpetic neuralgia but unfortunately will not completely prevent it from occurring.14 It is important to be aware of this syndrome, as it can cause debilitating pain and increase the risk of suicide from emotional distress.15
Bacterial infection is another common cause of infiltrative keratitis, with a major factor being contact lens use. The risk for infection with contact lens wear increases with overnight wear, poor case hygiene or storage, inadequate lens disinfection and poor hand washing prior to insertion and removal.16 Roughly 80% of bacterial keratitis cases are caused by Staphylococcus, Streptococcus and Pseudomonas species, but clinicians should also be wary of other causes such as Neisseria, Haemophilus, Corynebacterium diphtheriae, Neisseria meningitidis and Listeria as these species of bacteria can penetrate an intact corneal epithelium.16
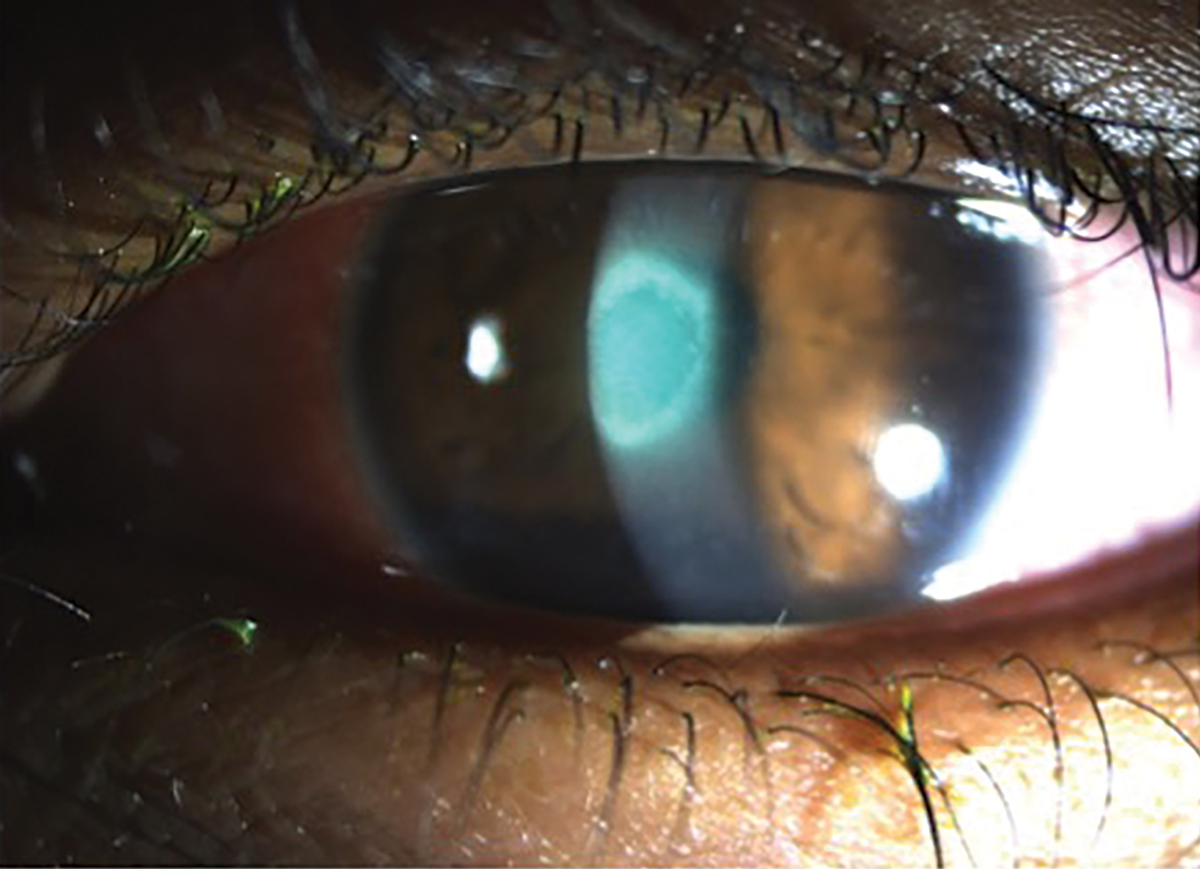
The presentation for bacterial keratitis may vary based on the organism, but most patients will present with blurred vision, pain, redness, discharge and/or photophobia.16 These symptoms will often coincide with the presence of a corneal ulcer secondary to a loss of epithelial tissue at the site of infection (Figure 6). This will then lead to a stromal infiltration of white blood cells such as polymorphonuclear and lymphomononuclear cells, which can lead to the destruction of Bowman’s layer and also stromal necrosis if the infection is left untreated.16 As the cornea tries to regenerate the epithelium, increase vascularization and remodel the stroma, this can ultimately lead to future neurotrophic changes as aberrant nerve regeneration may occur.
Drug toxicity. When prescribing any topical medication, patients must be provided with a thorough review of possible adverse reactions. These are an unintended or undesired effect of the drug that may occur from regular use of the medications, misuse of medications or overdose. There are many topical medications that can cause corneal toxicity.
 |
|
Fig. 5. This patient has a large area of epithelial disruption. There is a central epithelial defect and plaque, along with nasal neovascularization secondary to previous history of herpes zoster ophthalmicus keratouveitis and post-herpetic trigeminal neuralgia. Click image to enlarge. |
Topical antibiotics such as tobramycin or gentamycin are effective methods for treating bacterial conjunctivitis or corneal infections, but they have a common side effect of causing significant superficial punctate lesions to form, which can lead to discomfort and/or irritation.17 Topical anti-inflammatory medications such as NSAIDs or steroids can also cause issues with corneal epithelial wound healing and re-epithelization, especially when used together.17 Topical anesthetics, which are routinely used during exams, have been known to inhibit epithelial migration and destroy the superficial corneal epithelial microvilli.17 This can lead to loose epithelial cells and cause irritation amongst patients. Additionally, the preservatives in many over-the-counter drops such as artificial tears can cause damage to the epithelial cell; chlorobutanol and benzalkonium chloride are common culprits.17
These are just a few examples of corneal toxicity related to topical medications. Clinicians should be aware of the general side effects and the potential sequelae if there is corneal damage secondary to chronic or even abuse of a topical medication. Drug toxicity from routinely prescribed ocular medications can lead to neurotrophic changes and/or neuropathic pain, as damage to the corneal nerves from long term use of topical medications will affect corneal sensitivity.
Systemic disease. Diabetic neuropathy is a common complication of diabetes because of microvascular alterations.18,19 Many areas of the body can be affected—including the eye. Although we most frequently discuss the effects of diabetes on the posterior segment, specifically the retina, diabetes also impacts the anterior segment of the eye, including the cornea, conjunctiva and lacrimal glands.
Diabetes-induced corneal neuropathy is a common but underdiagnosed ocular complication of diabetes mellitus. Around 46% to 64% of patients develop diabetic neuropathic keratopathy throughout the clinical course of diabetes mellitus.18,19 The basic pathophysiology of diabetic corneal neuropathy is due to chronic hyperglycemia causing microvascular changes.18 These changes lead to local ischemia and poor nutrient delivery, resulting in progressive damage to the corneal nerves. Another factor is the inability of the damaged corneal nerves to secrete the neuromediators that help maintain ocular surface homeostasis.18
Early cases of diabetic corneal neuropathy may present as punctate epithelial erosions and tear film abnormalities.18,19 Moderate cases involve larger epithelial defects, RCEs and poor corneal wound healing.18,19 The most severe cases involve larger non-healing neurotrophic ulcers caused by reduced corneal sensation and potential corneal melt. 18,19 These more moderate and severe cases will be reflective of neurotrophic changes, as the corneal findings are more significant because of decreased corneal sensitivity. Therefore, it is imperative to consider diabetes in cases of both neurotrophic changes and neuropathic pain as the condition becomes more prevalent in the United States.
Trigeminal neuralgia is a sensory condition characterized by pain along one of the three branches of the nerve, characterized by an electric shock-like pain triggered by normally benign stimuli.20 It is the most common facial pain syndrome, with an annual incidence of four to 13 per 100,000.20 The ophthalmic branch (V1) innervates the scalp, upper eyelids, and cornea. Although the condition may be idiopathic, it is often caused by neurovascular compression. Isolated V1 trigeminal neuralgia occurs in 3% of patients.20 Ophthalmic manifestations include photophobia, lacrimation, excessive blinking and hyperemia.20
Fibromyalgia is a chronic systemic disorder characterized by widespread musculoskeletal pain, fatigue, sleep disturbances, headaches and cognitive issues.21 Most recently, it has been recognized as a neuropathic pain syndrome. Decreased small nerve fiber density has been found in patients with fibromyalgia in recent studies.21 The cornea has been found to have thinner stromal nerves and decreased sub-basal cell plexus nerve density when viewed with in vivo corneal microscopy in women with fibromyalgia.21 These nerve plexus changes have been associated with neuropathic pain. Anterior segment ocular features include severe pain and a higher incidence of dry eye syndrome.22
Treatments
Efforts to normalize corneal sensitivity will necessarily be tailored to the inciting condition or episode.
Neuroregenerative. Cenegermin 0.002% ophthalmic solution (Oxervate, Dompé) was approved by the FDA in 2018 for the treatment of moderate to severe neurotrophic keratitis.23 Cenegermin is a recombinant human nerve growth factor that has been shown to support corneal integrity through multiple mechanisms, including stimulating epithelial cell growth, limbal epithelial stem cell viability and the promotion of tear production via binding receptors on the lacrimal gland.23
Cenegermin has been studied in multiple clinical trials. Its efficacy in treating neurotrophic persistent epithelial defects compared to a vehicle sham over the course of eight weeks showed significantly improved reductions in lesion size.24 In achieving complete reduction of epithelial defects, cenegermin outperformed the vehicle sham at both the halfway point (four weeks) and eight-week endpoint.24 Cenegermin is a preservative-free ophthalmic solution dosed six times a day at two-hour intervals for eight weeks.23 While clinically effective, the dosing schedule and cost may be barriers to some patients.
 |
|
Fig. 6. A young patient presented with a new red and painful eye with light sensitivity secondary to bacterial keratitis from sleeping in monthly contact lenses. Click image to enlarge. |
Autologous serum tears, first reported in 1975 for treating tear deficiency in those with ocular surface disease and can successfully improve types related to systemic disease, ocular pathology and postoperative patients.25 Unlike artificial tears, blood-derived eye drops contain growth factors, platelets and proteins to help prevent cell death, improve cell growth, migration and promote healing.25
There are several scenarios where blood-derived drops such as serum tears, are useful, especially in cases of severe dry eye after maximum topical treatment, postsurgical complications with delayed healing, chronic epithelial defects and corneal neurotrophic pain. These serum tears can be written as a prescription to a compounding pharmacy and can be tailored to the patient with different concentrations depending on the ocular condition.
The blood is either drawn from the patient (autologous) or from a donor (allogenic), and the serum is then separated from the blood via a centrifuge to obtain a bright yellow, clear serum.25 The tubes are then combined with 0.9% sodium chloride to make the desired concentration, which varies according to clinician discretion starting at 20%.25 A 20% concentration tends to mimic the biological factors found in natural tears, which is why it is typically used in cases of dry eye, whereas concentrations of 40% to 50% are favored in corneal neuropathic pain to reduce photoallodynia by improving nerve density, decreasing nerve tortuosity and promoting epithelial healing.25
The dosage of serum tears may vary from TID to hourly, with the most common initial dose being four to eight times per day; many clinicians taper dosage over a few weeks depending of signs and symptoms of improvement.25 Patients may notice an improvement in symptoms in as little as two weeks, but it may take closer to four to six weeks to see a clinical change in some cases.25
Unfortunate drawbacks to serum tears may include inaccessibility to treatment, cost and storage of medication. The cost of treatment depends on location, dosage, concentration and associated lab/preparation fees, which are all typically not covered by insurance carriers, since the use of serum tears is not a FDA-approved therapy for ocular surface diseases.25
Amniotic membrane—another catalyst to healing—was first used in 1910 as a skin graft substitute material.26 Ophthalmic uses began in the 1940s as a conjunctival tissue substitute.26 Amniotic membranes are harvested from the innermost layer of the placenta. Thickness ranges from 20µm to 500µm and consists of multiple layers supported by a basement membrane.26 This intervention has unique biological properties including lack of immunogenicity, the ability to preserve and support stem cells and inhibition of inflammation, angiogenesis and fibrosis.26 In addition, improvement in pain and antimicrobial benefits have been observed.
Amniotic membranes come in different forms, typically cryopreserved or dehydrated. When placed on the cornea, dehydrated membranes are covered with a bandage contact lens (Figure 7). One study using cryopreserved amniotic membranes showed a decrease in neuropathic corneal pain from 6.3 to 1.9 (on a scale of 1 to 10) over a seven to 13-month follow-up period, with only two patients requiring repeat membrane placement.27 This makes amniotic membranes a versatile tool to use in conjunction with other topical therapies to promote good corneal healing in cases of both neurotrophic changes and neuropathic pain.
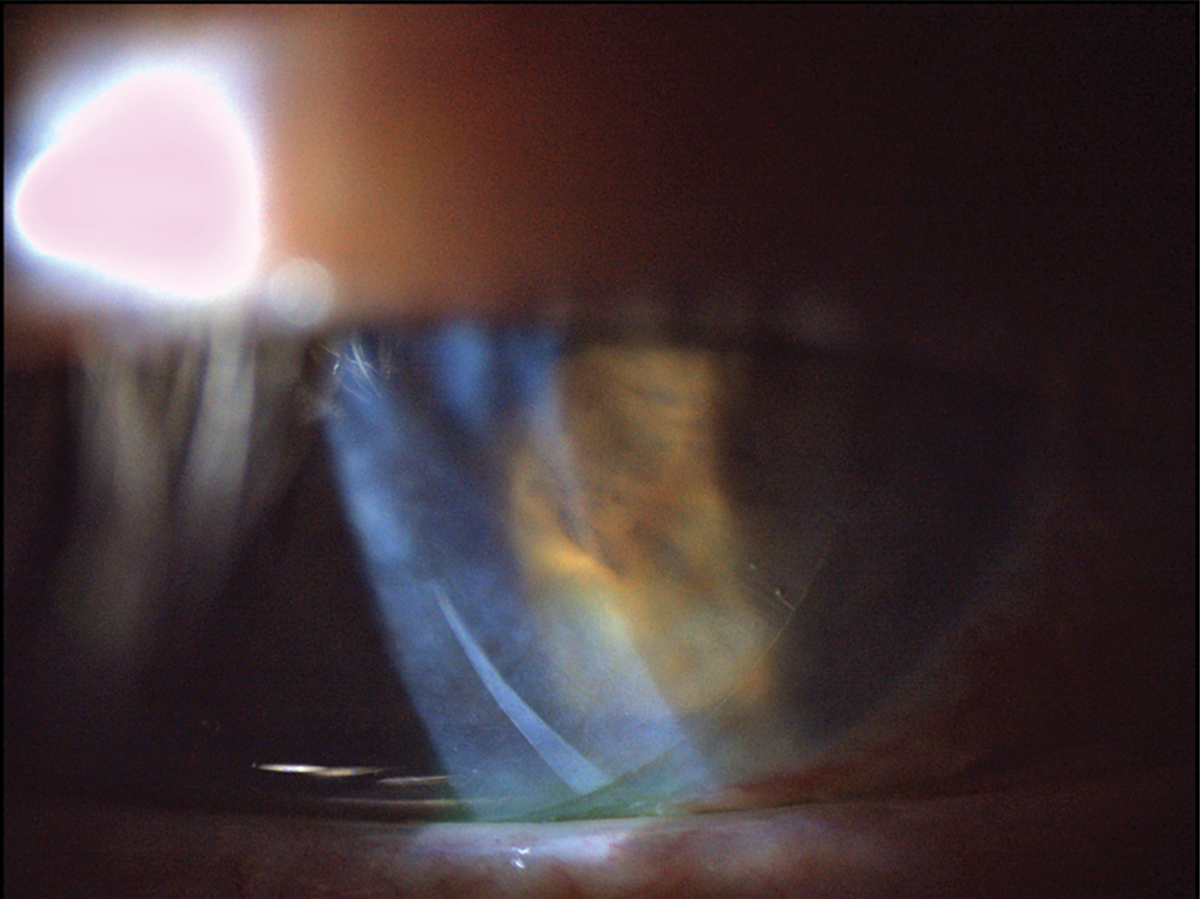 |
|
Fig. 7. This patient was fitted with a dehydrated amniotic membrane as seen underneath a bandage contact lens. There is mild folding of the dehydrated amniotic membrane as visible in the inferior cornea. Click image to enlarge. |
Anti-inflammatories. Corticosteroids are typically considered a first-line option for corneal pain because of their ability to provide rapid relief.28 These agents inhibit the activity of phospholipase A2, which is responsible for helping form prostanglandins and thromboxane A1 while also inhibiting other major portions of the inflammatory cascade to help reduce vasodilation, vascular permeability and stabilize the cell membrane.28 Typically, “soft” or low-dose steroids such as loteprednol or fluorometholone are used in cases where long-term treatment is needed, due to their reduced risks of IOP elevation and cataract formation compared with high-potency topical steroids such as prednisolone and difluprednate.
Topical cyclosporines are an immunomodulatory therapy that suppress the activation and function of T cells in order to reduce inflammation and relieve symptoms of ocular surface disease by downregulating inflammatory cytokines in the conjunctivae and lacrimal glands to improve conjunctival goblet cell density, increase tear production and decrease epithelial cell death.28,29 Typically, these medications are prescribed to those who have dry eye or inflammation related to an autoimmune condition. The regimen usually takes at least three weeks to three months to take effect and is most useful in controlling long-term chronic inflammation.
Another anti-inflammatory therapy is lifitegrast, (Xiidra, Bausch + Lomb), which decreases T-cell activation and recruitment by blocking the binding of intercellular adhesion molecule-1 to lymphocyte function-associated antigen 1.28 This helps prevent the migration, proliferation and release of many pro-inflammatory cytokines at the conjunctiva and lacrimal glands. It has a much faster onset of action at two weeks when compared with topical cyclosporine, which is good for cases of acute inflammation when faster relief is needed. The difference in time to efficacy is due to their different mechanisms of actions when limiting T cell-mediated inflammation.
These various options can be useful in conjunction with other neuro-regenerative therapies such as serum tears or cenegermin to help limit inflammation during the corneal nerve regeneration process.
Contact lenses. Long used to promote corneal wound healing and reduce ocular pain, bandage contact lenses form a physical barrier against the mechanical forces of the lid, which is helpful for reducing the risk of neurotrophic changes and neuropathic pain. Silicone hydrogel lenses allow for overnight wear and improve mechanical protection when compared with older lens materials; they are used for short-term relief from acute conditions such as RCE, trauma, post-surgical pain and chemical burns.30
Scleral lenses, which form a moisture chamber and fluid reservoir against the mechanical forces of the lid, are used in chronic ocular surface conditions when long-term bandage lenses are needed.
In most circumstances, therapeutic contact lenses are used in conjunction with topical drops such as artificial tears, anti-inflammatories and/or antibiotics. Clinicians should be mindful of risk factors related to extended contact lens wear, including corneal edema, neovascularization and stromal infiltrates. Routine lens checks are recommended.
Takeaways
Corneal neurotrophic changes and/or neuropathic pain can both be crippling ocular conditions, and it is essential to be aware of how they change a patient’s quality of life. Corneal nerves are important, as they maintain integrity and are a source of neuropeptides that provide a trophic support and aid in mediating any healing response to the cornea. Optometrists should perform a thorough case history, an ocular surface work-up, administer an ocular pain survey and consider additional testing like corneal esthesiometry or the proparacaine challenge test to monitor for corneal nerve changes.
With recent advancements in therapeutics, clinicians now have a wide variety of treatment options to choose from that include neuroregenerative and anti-inflammatory therapies to help control and manage both neurotrophic changes and neuropathic pain. Hopefully, these conditions will become more well understood as different testing methods and treatment options continue to develop in order to help patients overcome corneal nerve damage.
Dr. Grangaard is a staff optometrist and serves as the co-residency director at the Chillicothe VA Medical Center. He graduated from The Ohio State University College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is a fellow of the American Academy of Optometry and board-certified in medical optometry.
Dr. Hileman is the chief of the Optometry Service at the Chillicothe VA Medical Center. He graduated from Illinois College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is board-certified by the American Board of Certification in Medical Optometry.
Dr. Njeru is a staff optometrist at the Chillicothe VA Medical Center. He graduated from The Ohio State University College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is a fellow of the American Academy of Optometry. They have no financial disclosures.
1. Moshirfar M, Benstead EE, Sorrentino PM, et al. Ocular neuropathic pain. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK542282/. Last updated August 25, 2023. Accessed March 15, 2024. 2. Goyal S, Hamrah P. Understanding neuropathic corneal pain--gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31(1-2):59-70. 3. Mehra D, Cohen NK, Galor A. Ocular surface pain: a narrative review. Ophthalmol Ther. 2020;9(3):1-21. 4. Qazi Y, Hurwitz S, Khan S, et al. Validity and reliability of a novel Ocular Pain Assessment Survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology. 2016 Jul;123(7):1458-68. 5. Merayo-Lloves J, Gómez Martín C, Lozano-Sanroma J, et al. Assessment and safety of the new esthesiometer BRILL: comparison with the Cochet-Bonnet esthesiometer. Eur J Ophthalmol. October 29, 2023. [Epub ahead of print]. 6. Mead OG, Tighe S, Tseng SCG. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10(1):13-21. 7. Balal S, Ansari AS, Sim PY, et al. The incidence and prevalence of recurrent corneal erosion syndrome in London, UK. Eye (Lond). 2023;37(15):3213-6. 8. Lin SR, Aldave AJ, Chodosh J. Recurrent corneal erosion syndrome. Br J Ophthalmol. 2019;103(9):1204-8. 9. Moshirfar M, Bhavsar UM, Durnford KM, et al. Neuropathic corneal pain following LASIK Surgery: a retrospective case series. Ophthalmol Ther. 2021;10(3):677-89. 10. Bizrah M, Yusuf A, Ahmad S. An update on chemical eye burns. Eye (Lond). 2019;33(9):1362-77. 11. Ahmad B, Patel BC. Herpes simplex keratitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. pubmed.ncbi.nlm.nih.gov/31424862/. Last updated January 25, 2024. Accessed March 15, 2024. 12. Zhu L, Zhu H. Ocular herpes: the pathophysiology, management and treatment of herpetic eye diseases. Virol Sin. 2014;29(6):327-42. 13. Tsatsos M, MacGregor C, Athanasiadis I, et al. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016;44(9):824-37. 14. Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation and morbidity. Ophthalmology. 2008;115(2 Suppl):S3-12. 15. Kaufman SC. Anterior segment complications of herpes zoster ophthalmicus. Ophthalmology. 2008;115(2 Suppl):S24-32. 16. Singh P, Gupta A, Tripathy K. Keratitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. pubmed.ncbi.nlm.nih.gov/32644440/. Last updated August 25, 2023. Accessed March 15, 2024. 17. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25(10):1133-8. 18. So WZ, Qi Wong NS, Tan HC, et al. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen Res. 2022;17(10):2172-8. 19. Priyadarsini S, Whelchel A, Nicholas S, et al. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol. 2020;65(5):513-29. 20. Jones MR, Urits I, Ehrhardt KP, et al. A comprehensive review of trigeminal neuralgia. Curr Pain Headache Rep. 2019;23(10):74. 21. Ramírez M, Martínez-Martínez LA, Hernández-Quintela E, et al. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin Arthritis Rheum. 2015;45(2):214-9. 22. D’Souza S, Khamar P, Shetty R. Fibromyalgia syndrome and the eye—implications in corneal ultrastructure on confocal microscopy. Indian J Ophthalmol. 2023;71(4):1656-7. 23. Sheha H, Tighe S, Hashem O, et al. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973-80. 24. Pflugfelder SC, Massaro-Giordano M, Perez VL, et al. Topical recombinant human nerve growth factor (Cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. 2020;127(1):14-26. 25. Rojas L. Real-world uses of autologous serum eye drops. Rev Optom. 2023;160(11):58-65. 26. Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057-72. 27. Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16(1):132-8. 28. Grant R. How—and why—to choose dry eye drugs. Rev Optom. 2020;157(3):54-9. 29. de Paiva CS, Pflugfelder SC, Ng SM, et al. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst Rev. 2019;9(9):CD010051. 30. Sun YZ, Guo L, et al. Curative effect assessment of bandage contact lens in neurogenic keratitis. Int J Ophthalmol. 2014;7(6):980-3. |
