Can You Identify These Vitreous Anomalies?
The vitreous is critical to ocular diseases and their prognoses. Learn the techniques to make examination a breeze, and better identify and manage common aberrations.
Release Date:
June 2016
Expiration Date:
June 15, 2019
Goal Statement:
Although the vitreous humor comprises the largest ocular cavity, it is an optically clear structure, which makes it difficult to routinely examine. This course provides an overview on examination techniques as well as the identification and management of common vitreous anomalies. It also emphasizes the importance of careful examination of the vitreous, as this structure plays an integral role in the pathogenesis and prognosis of many retinal disorders.
Faculty/Editorial Board:
Bisant A. Labib, OD
Credit Statement:
COPE approval for 2 hours of CE credit. COPE ID is 50049-PS. Check with your local state licensing board to see if this counts toward your CE requirement for relic ensure.Disclosure Statement:
The authors have no relationships to disclose.
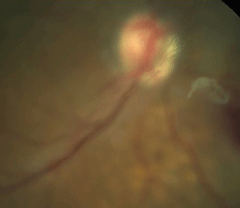 |
| Fig. 2. Fundus photograph of posterior vitreous detachment. |
Recent emphasis has been placed on the role of the vitreous in the prognosis of retinal disorders such as macular degeneration, making its examination of great clinical value. With its high concentration of water and its transparency, this important structure is often under examined and thus the identification of its various pathologies may be overlooked.
The Aging Vitreous
At birth, vitreous composition is made up of a rigid, hydrophilic and homogenous gel containing more than 98% water, as well as a matrix of collagen fibrils, hyaluronic acid (HA), proteoglycans (PGs) and glycoproteins (GPs).1,7 It is the precise spacing of the collagen and HA network that creates the structure of the gel, allowing for its transparency and strong adherence to the underlying retina.8
Liquefaction of the vitreous (synchysis) occurs as we age, and may begin as early as age four as evidenced through ultrasonographic studies; more than 50% of the vitreous liquefies by 80 years of age.9,10 The process of liquefaction is multifactorial and not well understood. Several of these mechanisms include damage wrought by oxidative stress through free radical formation, mechanical stress from ocular movements and enzymatic breakdown of the collagen meshwork.1,11-13
Vitreous syneresis (condensing or collapsing of the gel) concurrently occurs with age, and is what ultimately causes weakening of vitreoretinal adhesion at multiple sites. These processes occur over several years to decades.4
Age-Related Posterior Vitreous Detachment
The most common age-related vitreous change is the formation of a posterior vitreous detachment (PVD). This results from two insidious processes occurring at the vitreoretinal interface. The first is synchysis (as described above; an increase in gel liquefaction), which appears as collagen-free zones of fluid-filled pockets.7,9,11,14 The second is syneresis (the breakdown of the collagen arrangement), which manifests as optically dense areas within the vitreous chamber.7,14
Techniques to Examine the VitreousEye doctors of the past used to look right through the nearly transparent vitreous with hardly a second thought. Now, advances in imaging technology show us that the vitreous is an important ocular structure that has an integral role in a number of retinal diseases. These technologies include: • Slit lamp biomicroscopy. Routine slit lamp examination of the anterior vitreous includes static and dynamic observation to visualize the changes in the posterior cavity. Dynamic observation allows examination of the vitreous when the cortex is displaced and before it returns to its original position, which is important in the detection of pigment in the vitreous (Schaeffer’s sign), suggestive of a retinal break.3 Static vitreous examination is reliant on the Tyndall effect, which requires maximum pupil dilation and dark adaptation.4 To examine the posterior vitreous, use of a +90D lens increases the field of view to allow examination of the peripheral retina. • Indirect ophthalmoscopy. This method allows for an extended field of view as well as stereopsis, but due to the reduced image size, only significant alterations in the vitreous are readily visible. It is also sometimes difficult to retain binocularity when viewing the far retinal periphery.3 • Contact lens biomicroscopy. A three-mirror Goldmann lens allows the examiner to view the entire vitreous cavity. A narrowed beam aids in minimizing the amount of glow from the underlying choroi- dal vasculature.3 • B-scan ultrasonography. In the presence of ocular media opacifications, this method uses echography to detect vitreous opacities, membranes, and areas of adhesion and traction.3 In cases where vitreous abnormalities obscure the posterior segment of the eye, B-scan is useful in the timing and visualization of vitreoretinal surgeries.5 • Optical coherence tomography. While OCT is commonly used for macular disease and glaucoma progression, it also offers clinicians much information on areas of vitreous attachment, traction and detachment. This is most beneficial in the detection of abnormalities at the vitreoretinal interface, such as both vitreomacular and vitreopapillary adhesion and traction. With more advanced spectral domain imaging and the use of radial scans, the stages of posterior vitreous detachment are more readily understood.6 • Magnetic resonance imaging. MRI is often used in eye care for identification of neurological disorders, but may also assist in diagnosis of vitreous abnormalities especially in patients with hazy ocular media. MRI is useful in developmental anomalies, such as persistent hyperplastic primary vitreous, as well as vitreous hemorrhage and liquefaction.4 |
The combination of liquefaction with the aggregation of collagen fibrils results in weakening of the adhesion between the posterior vitreous cortex (PVC) and internal limiting membrane (ILM) of the retina at the posterior pole.9 This then allows liquefied vitreous to enter the retrohyaloid space through either microbreaks in the thin perifoveal layer of vitreous cortex or through the prepapillary hole in the vitreous cortex.9,15 Detachment begins first in the perifoveal region, and progresses to the superior and temporal midperiphery through gravitational effects. It then continues into the fovea, inferior midperiphery and finally to the level of the optic disc.9 Once this process is complete, a PVD is evident clinically in the form of a visible Weiss ring.
Several classifications or stages of PVD exist, depending mainly on the method of examination, which differentiate between a complete or partial PVD, depending on degree of attachment of the cortex to the retina. In general, the first stage is an incomplete perifoveal PVD in up to three quadrants. Stage two is the progression of perifoveal PVD in all quadrants with residual attachment to the fovea, optic nerve and midperipheral retina. Stage three consists of an incomplete PVD over the posterior pole with residual attachment to the optic nerve and midperipheral retina. Finally, stage four is identified as a complete detachment.14,16,17
The incidence of PVD increases with age and myopia.18-20 Some 63% of patients have a PVD by their eighth decade, with an estimated onset of 10 years earlier in myopes. Other factors accelerating this process include the presence of collagen vascular diseases (e.g., Marfan syndrome or Stickler syndrome), retinal vascular disease, trauma and inflammation.9
Recent studies establish a link between early onset PVD in postmenopausal women due to the potential effect of decreased estrogen and subsequent decrease in hyaluronic acid synthesis.9 PVDs are also sometimes induced following intravitreal injections, regardless of the injected agent, with a high incidence of PVD following cataract extraction.21
 |
| Fig 2. Fundus photograph of posterior vitreous detachment. Click image to enlarge. |
Floaters are the most commonly reported symptom of an age-related, non-pathologic PVD and result from either the aggregation of collagen into visible fibers, blood in the vitreous cavity or the glial remnant (Weiss ring) following detachment around the optic disc.20,22 The onset of a large amount of floaters with or without associated flashes indicate a concurrent retinal break from vitreous traction at the time of detachment.23 Flashes are reported in 50% of cases, occurring in mesopic conditions and mainly restricted to the temporal field.16 The occurrence of flashes is less understood, but they are likely due to the vitreous traction, and the separation of vitreous and retina induced by eye movements.20
Asteroid HyalosisAsteroid hyalosis (AH) is the most common clinically observed degenerative opacification of the vitreous.47 It manifests as small, cream-colored or white spherical bodies suspended in the vitreous cavity in either a random arrangement or deposited along the collagen fibrils.48-51 Under diffuse illumination, these asteroid bodies appear gold and are located predominantly in the inferior quadrant, oscillating with eye movement.51 The prevalence of AH is 1.0% to 1.2% of the population, predominantly in males, with a well-established link to increasing age.47,50- 52 AH presents unilaterally in 90% of cases and rarely impacts visual acuity.48,50,53 The correlation between AH and systemic conditions remains controversial. Earlier reports claimed an association between AH and diabetes, hypertension, hyperlipidemia, hyperopia, gout and increased serum calcium.50,52 However, the Beaver Dam Eye Study did not substantiate these claims.50 One report speculated that bilateral cases of AH have a statistically significant association in the diabetic population.47 While systemic associations remain inconclusive, anatomical analysis revealed a decrease in vitreous gel liquefaction and lower prevalence of complete PVD in patients with AH.54 Additionally, AH patients who do experience PVD will have a spontaneous, anomalous PVD and associated vitreoschisis. This is due to the presence of abnormal, firm vitreoretinal adhesion.54 |
Although the development of a non-pathologic, age-related PVD is a benign condition, symptomatic floaters have been associated with a marked decrease in contrast sensitivity and quality of life, giving rise to the investigation of treatment modalities for symptomatic patients.10,22 Nd:YAG laser has been used to treat the dense collagen fibers and large vitreous opacities that interfere with the visual axis, although studies reporting efficacy are inconclusive due to small sample sizes.10 Surgical vitrectomy has also been evaluated in these symptomatic patients, resulting in small improvements in visual acuity and symptomatology.10,22 Although minimal improvement is possible, surgical intervention is not without risk of complications, such as cataract development.22
Anomalous PVD
An anomalous PVD occurs when a portion of the posterior vitreous cortex remains attached to the internal limiting membrane of the retina.16,17 The most common site of partial detachment, before progressing further and detaching fully, is the superior retina.17 While complete PVDs commonly result from the age-related processes discussed above, an anomalous PVD may arise in cases where there is vitreous liquefaction without simultaneous dehiscence of the vitreoretinal interface.8,9,15 This causes traction at the interface, resulting in increased risk for developing retinal breaks and detachments, epimacular membrane formation, macular holes and vitreomacular traction.9
Complications vary depending on the location of the remaining area of adhesion. If liquefaction occurs with firm retinal adhesion in the periphery, a greater likelihood exists for retinal tears and detachments to develop. Remaining areas of adherence to the optic disc are more likely to promote vitreous hemorrhage or stimulate neovascularization.14 Finally, if attachment remains in the macular region, vitreomacular traction leading to macular hole and epimacular membrane formation is more likely to arise.14
Anomalous posterior retinal detachment occurs in approximately 26% of cases of vitreous detachment. The incidence is even greater in patients with lattice degeneration due to the strong adherence of vitreoretinal forces at the border of lattice lesions.8 As such, these patients should be followed for up to two years after initial diagnosis of incomplete and symptomatic PVD to assess vitreoretinal status.20
Other risk factors leading to anomalous posterior vitreous detachment include increased myopia (greater than -6.00D), history of trauma or surgery, other peripheral retinal degenerations (e.g., retinoschisis), and a personal or family history of retinal detachments.9,23
A higher frequency of complications also exists in pseudophakes or aphakes, those who experience subjective visual loss, and those with certain posterior segment findings such as retinal or vitreous hemorrhage, lattice degeneration, and the presence of “tobacco dust” in the anterior vitreous.23 The incidence of retinal break formation following posterior vitreous detachment is estimated to be 8% to 15%, and as high as 92% in the presence of pigment within the vitreous cavity.18
While detachments of the posterior vireous have the potential to cause serious and sight-threatening complications, they can be protective in some cases. Without the vitreous attachment to the underlying retina, the development of neovascularization in proliferative disease, such as diabetic retinopathy, is limited.16 Studies have also reported the efficacy of surgical PVD in the reduction of macular edema due to diabetes, vein occlusion and macular degeneration.21 Also, the presence of complete PVD allows rhegmatogenous retinal detachments to progress much more slowly than in patients with intact vitreous.16
Chemical VitrectomyThe vitreous is bound to the retina by a matrix of collagen and proteins, including laminin and fibronectin.40 Ocriplasmin, an injectable form of human plasmin with proteolytic activity, works on these proteins to release adhesion. It has two main mechanisms: to induce vitreous liquefaction and subsequent separation.44 It is FDA approved for patients with symptomatic VMT and with small full-thickness macular holes (less than 400μm).38 Studies show resolution in 26.5% of patients with vitreomacular adhesion, and closure in 40.6% of patients with macular holes after a single injection.44,46 It was found to be most effective in younger, female populations who are phakic.46 Though much less invasive than surgical pars plana vitrectomy, ocriplasmin intravitreal injections are not without side effects. The most common reported side effects are vitreous floaters, eye pain, photopsia, decreased vision or acute vision loss, dyschromatopsia or worsening of macular hole. Most of these side effects are transient and resolve within two weeks of onset; they likely affect the retinal photoreceptors as evidenced by OCT findings of transient damage to outer retinal layers, with all cases resolving.38 |
Vitreoschisis
Because of the remaining firm vitreoretinal attachments present in anomalous PVD, a splitting of the PVC may occur during syneresis; this is known as vitreoschisis, where the vitreous collapses forward and leaves the outermost layers adherent to the macula.14
In one study, 57% of patients with anomalous PVD exhibited concurrent vitreoschisis as evidenced through ultrasonographic and histopathologic studies.24 This is possible because of the anatomy of the vitreoretinal interface. It is now well recognized that several adhesion molecules, known as the “five-substance glue,” are responsible for the direct apposition of the vitreous to the retina. This matrix consists of fibronectin, laminin, opticin, chondroitin sulfate and heparin sulfate, all of which are located between the PVC and ILM and are responsible for holding the collagen fibrils in place.14,25
Also keep in mind the anatomy of the PVC, which is a multi-lamellar structure containing a single layer of hyalocytes. Due to the PVC’s multi-lamellar composition, a vitreoschisis may occur at any level, and causing different degrees of complications.25
Vitreoschisis is a well-known finding in the development of proliferative diabetic retinopathy (PDR), occurring in 80% of cases.14,24 Vitreoschisis is also prevalent in macular disease, specifically 53% of macular holes and 43% of macular pucker.14,24-26 The occurrence of the schisis in reference to the layer of hyalocytes is presumed to play an integral role in macular hole formation in comparison with macular pucker. If the schisis occurs posterior to hyalocyte layer in the PVC, the anterior surface of the cortex will detach along with the detachment of hyalocytes, forming a macular hole. In contrast, schisis that occurs anterior to the hyalocyte layer will leave remnant hyalocytes adhering to the macula, forming a macular pucker.26
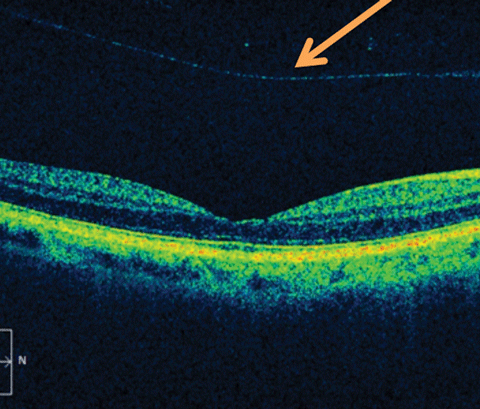
 |
| Fig. 3. OCT of an epiretinal membrane, a complication of anomalous PVD. Click image to enlarge. |
Besides anomalous posterior vitreous detachment, vitreoschisis may also be induced by vitreoretinal surgery, leading the surgeon to take extra care to search for membranes during peels. If removal of the PVC is incomplete following surgical intervention, it may give rise to cases of tractional retinal re-detachment and epiretinal membrane reformation.14 As such, alterations of vitreoretinal surgical techniques are currently being evaluated.
Vitreous Hemorrhage
Proliferative disease, notably proliferative diabetic retinopathy (PDR), is the main cause of blood in the vitreous cavity, known as vitreous hemorrhage (VH). Other identifiable causes of VH include trauma, age-related macular degeneration (AMD), vein occlusion, sickle cell retinopathy and PVD. In an estimated 8.3% of cases, the underlying cause remains unidentifiable, even following surgical vitrectomy.27 The prevalence of acute onset VH is seven cases for every 100,000 people.27
The etiology of VH is important to ascertain as it often dictates the visual prognosis and management. Most patients younger than 60 have a VH secondary to a retinal tear, which releases blood into the vitreous cavity. Because the sites of vitreous attachments include retinal blood vessels, traction in this region can also release blood into the vitreous cavity. Patients with this etiology have good visual resolution and outcome without the need for surgical intervention.27 In contrast, patients older than 60 years of age most commonly present with acute VH secondary to proliferative disease, such as PDR, AMD, sickle cell retinopathy and venous occlusion.27
Retinal ischemia results in hypoxia, which leads to the development of neovascularization that invades the space between the retinal and posterior vitreous, leading to traction and subsequent VH.28 Patients with this known cause of VH have poorer visual outcome. Laser photocoagulation in conjunction with an anti-VEGF agent is recommended, and early pars plana vitrectomy in some cases is also warranted.27,28
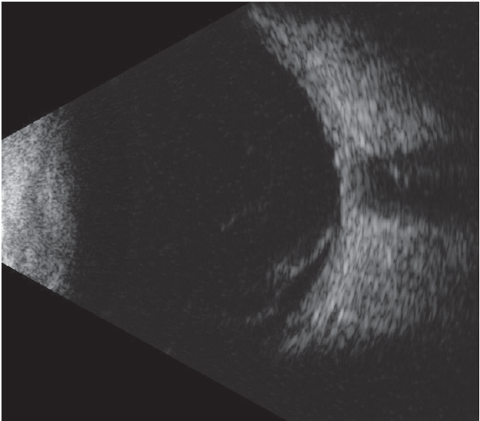 |
| Fig. 4. B-scan image of a vitreous hemorrhage. |
Other, less common causes of VH include use of oral anticoagulants, radiation retinopathy, Terson’s syndrome, anomalous PVD and carbon monoxide poisoning.29-33 Rarely, VH can occur secondary to Ozurdex implant, iridocilliary cyst and from optocilliary vessels following chronic papilledema.34-36
In children with bilateral VH, it is important to first consider shaken baby syndrome, as trauma is the leading cause of the presentation in pediatric patients. Vasculitis secondary to systemic disease such as tuberculosis may also result in VH formation. Other conditions to consider are hematologic disorders, including leukemia, sickle cell anemia and thrombocytopenia. Less commonly, VH may arise from the presence and leakage of a persistent hyaloid artery. Better visual outcomes in children are found in cases of closed-globe injuries and vascular and hematologic causes.37
Vitreomacular Traction
When the vitreous begins to detach without subsequent separation from the macula, these vitreomacular adhesions may progress and cause morphological disturbances to the underlying retinal surface, known as vitreomacular traction (VMT). This manifests as a symptomatic vision decrease or metamorphopsia.38,39 VMT may give rise to complications such as cystoid macular edema (CME), macular pucker, tractional macular detachment, epiretinal membrane or macular hole formation.40
The prevalence of VMT is 0.35% to 1.6% of the population and has been implicated in multiple disease processes, including diabetic macular edema, AMD, venous occlusion and myopic traction maculopathy.38,41
VMT is classified in two main categories, broad or focal, depending on the width of vitreous attachment to the retina. Broad areas of attachment can lead to generalized macular thickness, vascular leakage on fluorescein angiography, macular schisis and cystoid macular edema. Focal attachments may elevate the foveal floor or cause pseudocysts in the central macula.42
Anatomical configuration and clinical course of VMT vary greatly among individuals, making it difficult to establish a treatment standard.41 Diagnosis is made through structural changes on OCT in three stages: evidence of perifoveal vitreous cortex detachment from the retinal surface; macular attachment of the vitreous cortex within a 3mm radius of the fovea; and finally, association of attachment with distortion of the foveal surface, intraretinal structural changes, elevation of the fovea above the RPE, or a combination of these. No full-thickness interruption of the retinal layers exists.41
For patients with symptomatic VMT, pars plana vitrectomy is a well-documented surgical treatment. While effective, this form of intervention is invasive and has the ability to cause serious complications like cataracts, retinal detachment or endophthalmitis.43 In 2012, ocriplasmin injection (Jetrea, ThromboGenics) was approved for the treatment of symptomatic VMT and small, full-thickness macular holes. This serine protease enzyme induces vitreous liquefaction by lysing molecular substrates connecting the vitreous to the retina, and releasing adhesion at the macula and peripapillary retina, with fewer side effects than more invasive techniques.38,40,44 (See “Chemical Vitrectomy”)
Another study has suggested the potential use of a single intravitreal injection of C3F3 gas in patients with symptomatic and persistent VMT. Release occurred in 40% of patients within one month, and in up to 60% of cases at six-month follow up.45
Research has also shown that patients receiving intravitreal anti-VEGF injections can have spontaneous resolution of VMT, especially when retinal distortion is limited to the inner retinal layers. As such, it is best to watch these patients not only because of the higher rate of spontaneous resolution, but because removing the vitreous using pars plana vitrectomy will make the injected agents less effective.41
Although formerly known as the “vitreous humor”—one of several bodily fluids or “humors”—there’s nothing humorous about the important involvement of the vitreous in a plethora of serious retinal diseases. Advanced research and improved instrumentation have now given us a clearer view of this easy-to-overlook but very important structure.
Dr. Labib is a full-time assistant professor at the Pennsylvania College of Optometry at Salus University, in Elkins Park, Pa., working in both the traditional optometry program as well as the accelerated scholars program.
|
1. Kodama M, Matsuura T, Hara Y. Structure of vitreous body and its relationship with liquefaction. J Biomedical Science and Engineering. 2013;6:739-745. 2. Gao Q, Fu Y, Hui Y. Vitreous substitutes: challenges and directions. Int J Ophthalmol. 2015;(8)3;437-440. 3. Schepens CL, Neetens A. The Vitreous and Vitreoretinal Interface. New York: Springer-Verlag, 1987. Print. 4. Sebag J. Vitreous in Health and Disease. New York. 2014. Print. 5. Ahmed J, Shaikh FF, Rizwan A, Memon MF. Evaluation of vitreo-retinal pathologies using B-scan ultrasound. Pak J Ophthalmol. 2009;25(4):1-4. 6. Pang CE, Freund KB, Engelbert M. Enhanced vitreous imaging technique with spectral-domain optical coherence tomography for evaluation of posterior vitreous detachment. JAMA Ophthalmol. 2014; 132(9);1148-50. 7. Los LI, Van der Worp RJ, Van Luyn MJ, Hooymans JM. Age-related liquefaction of the human vitreous body: LM and TEM evaluation of the role of proteoglycans and collagen. Invest Ophthalmol Vis Sci. 2003;44(7):2828-33. 8. Carrero JL. Incomplete posterior vitreous detachment: prevalence and clinical relevance. Am J Ophthalmol. 2012;153(3):497-503. 9. Johnson M. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010 Mar;149(3):371-82. 10. Milston R, Madigan M, Sebag J. Vitreous floaters: etiology, diagnostics, and management. Surv Ophthalmol. 2016 Mar-Apr;61(2):211-27. 11. Nuzzi R, Marchese A, Gulino GR, et al. Influence of posterior detachment and type of intraocular lens on lipid peroxidation in the human vitreous. Mol Vis. 2015 Sep;21(3):1106-1112. 12. Bonfiglio A, Lagazzo A, Repetto R, et al. An experimental model of vitreous motion induced by eye rotations. Eye and Vision. 2015;2(10):1-10. 13. Beebe DC, Holekamp NM, Siegfried C, et al. Vitreoretinal influences on lens function and cataracts. Phil Trans R Soc B. 2011;366:1293-1300. 14. Romano M, Comune C, Ferrara M, et al. Retinal changes induced by epiretinal tangential forces. J Ophthalmol. 2015;2015:372564. 15. Kicova N, Bertelmann T, Irle S, et al. Evaluation of a posterior vitreous detachment: a comparison of biomicroscopy, B-scan ultrasonography and optical coherence tomography to surgical findings with chromodissection. Acta Ophthalmologica. 2012;90:264-268. 16. Kakehashi A, Takezawa M, Akiba J. Classification of posterior vitreous detachment. Clin Ophthalmol. 2014;8:1-10. 17. Zarbin M, Chu D. Optical coherence tomography use in evaluation of the vitreoretinal interface: a review. Surv Ophthalmol. 2007;52(4):397-421. 18. Tanner V, et al. Acute posterior vitreous detachment: the predictive value of vitreous pigment and symptomology. Br J Ophthalmol 2000;84:1264-1268. 19. Ma F, et al. Optical coherence tomography findings of the vitreoretinal interface in asymptomatic fellow eyes of patients with acute posterior vitreous detachment. Retina. 2014;34:447-454. 20. Goh YW, Ehrlich R, Stewart J, et al. The incidence of retinal breaks in the presenting and fellow eyes in patients with acute symptomatic posterior vitreous detachment and their associated risk factors. Asia Pac J Ophthalmol 2015;4:5-8. 21. Geck U, Pustolla N, Baraki H, et al. Posterior vitreous detachment following intravitreal drug injection. Graefes Arch Clin Exp Ophthalmol. 2013;251:1691-1695. 22. Sebag J, Yee K, et al. Vitrectomy for floaters prospective efficacy analyses and retrospective safety profile. Retina. 2014;34;1062-1068. 23. Schweitzer K, Eneh A, Hurst J, et al. Predicting retinal tears in posterior vitreous detachment. Can J Ophthalmol. 2011;46:481-485. 24. Sebag J. Vitreoschisis in diabetic macular edema. Invest Ophthalmol Vis Sci. 2011 Oct;10(52):8455-6. 25. Sebag J. Vitreoschisis. Graefes Arch Clin Exp Ophthalmol. 2008;246:329-332. 26. Sebag J, Gupta P, Rosen R, et al. Macular holes and macular pucker: the role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Trans Am Ophthalmol Soc. 2007;105:121-131 27. Kim DY, Joe SG, Baek S, et al. Acute-onset vitreous hemorrhage of unknown origin before vitrectomy: causes and prognosis. J Opthalmol. 2015;6:1-8. 28. Annan J, Carvounis P. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int Ophthalmol Clin. 2014;54(2):141-153. 29. Jun JH, Hwang JC. Association of rivaroxaban anticoagulation and spontaneous vitreous hemorrhage. JAMA Opthalmology. 2015;133(10)1184-1186. 30. Montero JA, Yanez-Castro G, Sanchis-Merino ME, et al. Bevacizumab in vitreous hemorrhage secondary to radiation retinopathy. BMJ Case Rep. 2014:1-3. 31. Keradzic J, Kovacevic I, Stefanovic I, et al. Terson’s syndrome: a report of two cases. Srp Arh Celok Lek. 2015 SepOct;143(9-10):595-598. 32. Melamud A, Pham H, Stoumbos Z. Early vitrectomy for fundus-obscuring vitreous hemorrhage. Am J Ophthalmol. 2015;160(5):1073-1077. 33. Levin M, Hall J, Guerami A. Vitreous hemorrhage from carbon monoxide retinopathy. Retinal Cases Brief Rep. 2016 Spring;10(2):157-9. 34. Casati S, Bruni E, Marchani G. Retinal and vitreous hemorrhage after impact of dexamethasone implant in a vitrectomized eye. Eur J Opthalmol. 2015;12:1-7. 35. Rivero V, Aparicio MJ, Suarez-Leoz M, et al. Vitreous hemorrhage secondary to iridociliary cyst. Arch Soc Esp Oftalmol. 2015;90:600-603. 36. Fraser C, et al. Vitreous hemorrhage secondary to optociliary shunt vessels from papilledema. J Neuro Opthalmol. 2012;32:332-334. 37. Sudhalkar A, et al. Bilateral vitreous hemorrhage in children: clinical features and outcomes. J Opthalmic Vis Res. 2015;10(2):139-142. 38. Kaiser P, Kampik A, Kuppermann B, et al. Safety profile of ocriplasmin for the pharmacologic treatment of symptomatic vitreomacular adhesion/traction. Retina. 2015;35:1111-1127. 39. Ichiyama Y, et al. Photoreceptor outer segment length and outer foveal thickness as factors associated with visual outcome after vitrectomy for vitreomacular traction syndrome. Retina. 2016;0:1-6. 40. Tyler L, Singer M, Bell D. Long term outcomes in patients with vitreomacular traction treated with a single intravitreal injection of ocriplasmin. Retinal Cases Brief Rep. 2016 Feb 4. [Epub ahead of print]. 41. Duker J, Kaiser P, Binder S, et al. The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. American Academy of Ophthalmology. 2013(12);120:2611-2619. 42. Duker J, Kaiser P, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611-2619. 43. Odrobina D, Laudanska-Olszewska I, Gozdek P. Macular hole formation and spontaneous closure after vitrectomy for vitreomacular traction documented in spectral-domain optical coherence tomography. BMC Opthalmology. 2014:14-17. 44. Chatziralli IP, Theodossiadis GP, Parikakis E, et al. Complications of intravitreal ocriplasmin for vitreomacular traction and macular hole: a prospective spectral-domain optical coherence tomography study. Cutan Ocul Toxicol. 2015;10:1-7. 45. Rodrigues IA, Stangos AN, McHugh DA, et al. Intravitreal injection of expansile perifluoropropane (c(3)f(8)) for the treatment of vitreomacular traction. Am J Ophthalmol. 2013 Feb;155(2):270-276. 46. Chatziralli I, Theodossiadis G, Parikakis E, et al. Real life experience after intravitreal ocriplasmin for vitreomacular traction and macular hole; a spectral domain optical coherence tomography prospective study. Graefes Arch Clin Exp Opthalmol. 2016;254:223-233. 47. Kador PF, Wyman M. Asteroid hyalosis: pathogenesis and prospects for prevention. Eye Lond. 2008;22(10):1278-85. 48. Hwang JC, Barile GR, Schiff WM, et al. Optical coherence tomography in asteroid hyalosis. Retina. 2006;26(6):661-665. 49. Ikeda Y, Hisatomi T, Murakami Y, et al. Retinitis pigmentosa associated with asteroid hyalosis. Retina. 2010;30(8):1278-1281. 50. Moss S, Klein R, Klein B. Asteroid hyalosis in a population: the Beaver Dam Eye Study. Am J Opthalmol. 2001;132:70-75. 51. Galveia J, et al. Asteroid hyalosis – clinical review of 58 cases. Rev Bras Oftalmol. 2013;72(10):1-8. 52. Mitchell P, Wang M, Wang J. Asteroid hyalosis in an older population: the Blue Mountains Eye Study. 2003;10(5):331-335. 53. Yazar Z, Hanioglu S, Karakoc G, et al. Asteroid hyalosis. Eur J Ophthalmol. 2001;11(1):57-61. 54. Mochizuki Y, Hata Y, Kita T, et al. Anatomical findings of vitreoretinal interface in eyes with asteroid hyalosis. Graefes Arch Clin Exp Ophthalmol. 2009;247:1173-1177. |
