Antibiotics: Wonder Drugs For Treating Anterior Segment Infections
Selecting the appropriate medication is essential for optimal success when treating meibomian gland dysfunction, blepharitis and other anterior segment conditions.
Release Date:
February 2016
Expiration Date:
February 1, 2019
Goal Statement:
Safe and effective use of antibiotics is essential for safeguarding the ocular health of our patients. Whether treating meibomian gland dysfunction, blepharitis, bacterial conjunctivitis or any other infection in need of antibiotics, choosing the right medication to treat the suspected bacteria, while also avoiding sub-lethal dosing, are the keys to a successful treatment regimen. This article discusses antibiotic treatment options for common anterior segment infections.
Faculty/Editorial Board:
Chandra Mickles, OD, MS
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID is 48717-AS. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Dr. Mickles consults for Alcon and Allergan.
Antibiotics play a vital role in containing and eradicating sight-threatening eye conditions such as eyelid disease, bacterial conjunctivitis and bacterial keratitis. They are also widely used for prophylaxis. Safe and effective use of anti-infective medications is essential for safeguarding the ocular health of our patients. Choosing an antibiotic with antimicrobial efficacy against the suspected bacteria, as well as avoiding sub-lethal dosing, is paramount to success. This article reviews current and emerging antibiotic options for common anterior segment conditions necessitating anti-infective agents.
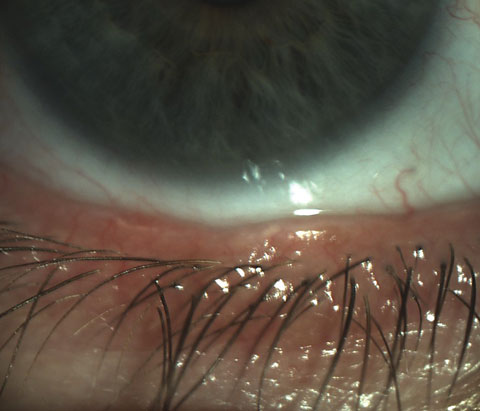 |
| Scalloped lid margins are indicative of inflammation in meibomian gland dysfunction. |
Beyond Lid Hygiene
Meibomian gland dysfunction (MGD) and blepharitis are among the most prevalent problems encountered in the clinical setting.1 While eyelid hygiene and warm compresses remain first-line therapies, topical and oral antibiotic options have expanded recently to better manage these chronic anterior segment conditions.
Although the etiologies of MGD and blepharitis are poorly understood, the toxic effects of bacteria that colonize the eyelid margin, as well as inflammation, are factors. Research suggests that the pathogenic process is associated with the release of toxic bacterial products that stimulate the production and release of pro-inflammatory cytokines.2 Therefore, macrolides are an ideal antimicrobial choice due to their antibacterial and anti-inflammatory activity.
Although the macrolide erythromycin has been the mainstay for these conditions, antibiotic resistance may limit its efficacy because of its extensive use.3 Azithromycin, a second-generation macrolide, has recently gained popularity because of its anti-inflammatory properties, excellent ocular tissue penetration and prolonged duration of activity.4 Topical azithromycin 1% (AzaSite, Akorn) increases retention time, which is believed to be due to its viscous carrier, polycarbophil, that allows for more contact time on the eyelid margin.
Although used off-label for MGD and blepharitis, best success is achieved for these conditions when AzaSite is administered to a closed eyelid with massage twice a day for two days, then daily for a month. Because AzaSite is not approved for patients less than one year of age, erythromycin or bacitracin ointment can be applied at bedtime for up to a month for these younger patients.
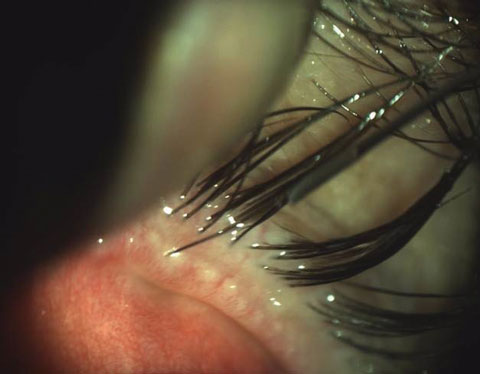 |
| Findings such as obstructed meibomian glands with telangiectasia indicate that conventional eyelid therapy may not be sufficient for MGD management. |
When Topicals Aren't Enough
Similar to topical treatments, oral antibiotics with antimicrobial and anti-inflammatory properties are excellent choices for managing refractory cases of MGD and blepharitis. Oral azithromycin and doxycycline are therapeutic options in these cases.5,6,7 While 50mg doxycycline per day for one to six months is typically used for MGD and blepharitis management, as low as 20mg BID for one month was shown to be equally effective as a four-week treatment of 200mg doxycycline BID.5 Oral azithromycin can be given as pulsed therapy (500mg per day over three consecutive days in each week for one month) or a five-day treatment of 500mg on day one and 250mg per day thereafter.
While both doxycycline and oral azithromycin have been shown to be effective in MGD and blepharitis treatment, knowing which oral antibiotic has better efficacy in each case can be challenging.5,7 For treating recalcitrant MGD, researchers found that the five-day oral azithromycin regimen had a significantly better clinical response and fewer side effects than doxycycline 200mg/day for one month.7 Oral azithromycin's better clinical response and shorter treatment duration suggest it may be the better oral antibiotic option for meibomian gland dysfunction. Investigators recently demonstrated that the greater efficacy of oral azithromycin may be due to its ability to stimulate the meibomian gland epithelial cell function, which is not duplicated by doxycycline and the other tetracyclines.8
Both topical and oral antibacterial therapies show promise for effectively managing eyelid disease. Practitioners should also keep in mind that maintenance therapy of eyelid hygiene and warm compresses may be needed in addition to antibiotics in some cases.
Winning the Battle Against Bacterial Conjunctivitis
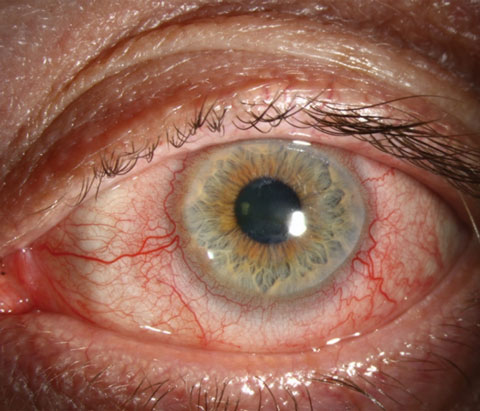 |
| Antibiotics can help manage the toxic effects of bacteria that colonize the eyelid margin and cause inflammation, as seen here with rosacea blepharitis. Photo: Christine W. Sindt, OD. |
Bacterial infection is a common cause of infectious conjunctivitis, which is characterized by hyperemia and purulent discharge.9 Although bacterial conjunctivitis is typically self-limiting, empiric antibiotic therapy can decrease disease transmission and severity, minimize complications and reduce recurrence.10
Besivance (besifloxacin 0.6% ophthalmic suspension, Bausch + Lomb) is a highly effective broad-spectrum antibiotic used TID for seven days for bacterial conjunctivitis. It is the first topical chlorofluoroquinolone developed solely for ophthalmic use, and it is effective even against multi-drug resistant Staphylococci.11 Although its lack of a systemic counterpart limits the likelihood of bacterial resistance, overuse is still a concern. Thus, newer generation antibiotics, such as besifloxacin, should be saved for moderate to severe cases of bacterial conjunctivitis.
The use of the earlier generation topical fluoroquinolones in milder cases has been supported, given bacterial conjunctivitis is usually self-limiting and the causative microbe of bacterial conjunctivitis has generally not been methicillin-resistant Staphylococcus aureus (MRSA).12 Other good alternatives for bacterial conjunctivitis management are topical azithromycin and polymyxin B/bacitracin ointment for children struggling with drop instillation.
Breaking Down Bacterial Keratitis
When faced with a suspected bacterial corneal ulcer, prompt institution of effective therapy is a must to maximize the chances of complete recovery. Unfortunately, this ocular emergency can rapidly cause corneal destruction, leading to potential vision loss. Bacterial keratitis is the most common form of microbial keratitis.13 Because both gram-negative and gram-positive bacteria are causative pathogens, use of an adequately dosed broad-spectrum antibiotic that covers both gram-positive and gram-negative microorganisms is preferred. Depending on the severity, around-the-clock application of besifloxacin is an excellent choice in most cases. Initially instilling the antibiotic drops in-office can demonstrate the importance of frequent dosing, potentially increase therapeutic compliance and ultimately improve patient outcomes.
 |
| Besifloxacin 0.6% ophthalmic suspension, the first topical chlorofluoroquinolone developed solely for ophthalmic use, is often used to treat mucopurulent bacterial conjunctivitis. Photo: Sherrol A. Reynolds, OD. |
The addition of dual broad-spectrum fortified antibiotics, usually a cephalosporin and an aminoglycoside, is indicated for central, large (>2mm) or aggressive bacterial corneal ulcers.14,15 Infrequently, systemic antibiotics are warranted for bacterial keratitis management. Systemic antibiotics and periocular injections of antibiotics should be considered if corneal perforation is imminent or the infection process has invaded the sclera.
Old Drug, New Trick
The emergence of bacterial resistance and the shortage of new anti-infective agents have driven the reconsideration of older antibiotics as viable therapeutic options. Colistin (Taj Pharmaceuticals) is a polymyxin drug that was used extensively systemically, but was abandoned due to side effects when given parenterally.16 However, there has been a resurgence in its use due to its activity against antibiotic resistant gram-negative bacteria.16-20 Topical colistin may be a valuable option for cases of multiple drug-resistant Pseudomonas bacterial keratitis. In a recent study, topical colistin 0.19% was found to be safe and effective against multi-drug resistant Pseudomonas aeruginosa.20
The Missing Link
The emergence of antibiotic-resistant pathogens has also prompted the search of alternative techniques that can effectively treat bacterial keratitis. Corneal collagen crosslinking (CXL) is a novel technique that slows the progression of keratoconus and postoperative ectasia. During the standard CXL procedure, the cornea is irradiated with ultraviolet A (UVA) light after application of the photosensitizer riboflavin.21 This process strengthens the corneal collagen, thereby stabilizing the cornea.
Research has shown riboflavin and UVA irradiation to be bactericidal against gram-negative and gram-positive bacteria, including MRSA.22,23 UVA has the ability to directly destroy bacteria, while the riboflavin can produce reactive oxygen species toxic to the microorganisms.24,25 CXL also has a toxic effect on these inflammatory cells, and it increases the cornea's resistance to enzymatic degradation.26,27 These actions should limit corneal scarring and prevent corneal perforation. Thus, CXL may be a useful alternative treatment option in severe or recalcitrant cases of bacterial keratitis. It may also be beneficial when compliance with conventional antibiotic treatment is an issue.
In several studies, adjunctive CXL therapy, or CXL alone, shortened the healing time and reduced the complication rate of bacterial keratitis when compared to topical antibiotic therapy alone.22,24,25,28,29 However, in a prospective trial comparing CXL plus conventional topical treatment with antibiotic treatment alone, researchers did not find a difference in healing time between the two groups.21 Nonetheless, they did find fewer complications in the group treated with both topical antimicrobial therapy and adjunctive CXL.21
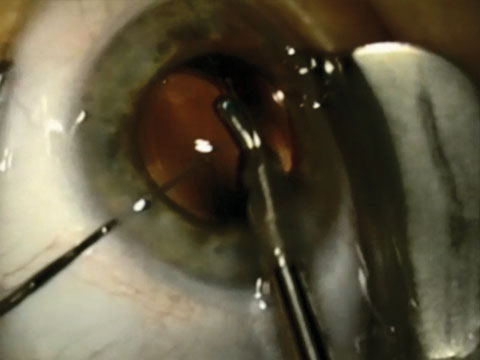 |
| Injecting medication into the posterior chamber after uncomplicated cataract surgery provides sustained therapeutic protection against endophthalmitis and postoperative cystoid macular edema. Photo: Richard Mangan, OD. |
Despite the reported success of CXL treatment for bacterial keratitis, there are potential safety concerns. The cornea must be a certain thickness for CXL to protect the corneal endothelium from damage, and measuring corneal thickness in the presence of active infection can be difficult.30 Additionally, it is unknown what effect a corneal infiltrate would have on the penetration of the riboflavin and UVA.31 There is not enough data showing the safety of CXL for bacterial keratitis treatment long-term, nor is there an established optimal protocol. Therefore, the use of CXL may be best reserved for severe bacterial keratitis or cases refractory to conventional antibiotic therapy.21,24,25
Adjunctive Steroids: Where Do We Stand?
The use of adjunctive topical corticosteroids in the treatment of bacterial keratitis has been a controversial issue in the eye care community. The potential benefits of the corticosteroid—minimizing scarring and ultimately improving visual outcomes—are juxtaposed with concern over exacerbation of infection and delayed healing. The Steroids for Corneal Ulcers Trial (SCUT) attempted to provide evidence for treatment practices for bacterial keratitis by assessing the effect of adjunctive corticosteroids on clinical outcomes.32 Five hundred participants with culture-positive bacterial keratitis received, as adjunctive therapy, either topical corticosteroids or topical placebo.33 The results revealed no significant difference in the scar size and visual outcomes between the steroid treatment group and the control group.33 However, sub-group analyses revealed a modest benefit in visual acuity in steroid-treated participants with central or deep ulcers.33
Following these results, questions remained about the long-term outcome of adjunctive steroid use. A 12-month SCUT follow-up study revealed that adjunctive corticosteroids may be associated with improved, long-term clinical outcomes for bacterial ulcers not caused by Nocardia species.32 The best spectacle-corrected visual acuity improved at 12 months for the steroid group.
However, in a recent report of visual outcomes four years following the initial SCUT study, the visual acuity did not significantly change between one year and four years post-treatment in a subset of 50 participants representative of the larger SCUT cohort.34 This suggests that bacterial keratitis treated with adjunctive steroids may yield improvements in visual acuity 12 months following diagnosis, but improvement thereafter is unlikely.
In light of these results, clinicians should not postpone surgical intervention with corneal transplantation beyond one year for bacterial keratitis cases.34 A conservative approach is to avoid corticosteroid initiation until the offending pathogen is identified and then provide close follow up if the corticosteroid is introduced. Judicious evidenced-based prescribing and prudent follow up is always warranted.
Prophylaxis
Antibiotic prophylaxis is also a topic of great debate in the eye care community. Questions remain as to whether or not topical antibiotics are overkill in peri-procedural prophylaxis and if clinicians are using antibiotics excessively in cases of superficial keratitis, viral conjunctivitis and corneal abrasions.
Topical antibiotics are administered perioperatively and peri-procedurally to prevent endophthalmitis. Studies have demonstrated that topical antibiotics, administered under these circumstances, significantly reduce conjunctival bacterial flora.35,36 However, there is increasing bacterial resistance to the frequently used topical fluoroquinolones. Thus, unwarranted overuse of topical fluoroquinolones for peri-procedural and perioperative prophylaxis is a concern.
 |
| A corneal ulcer's location is an important factor in treatment. While controversial, adjunctive corticosteroids may improve outcomes for central or deep bacterial corneal ulcers. Photo: Sherrol A. Reynolds, OD. |
The standard use of 5% povidone-iodine with meticulous sterile preparation is an effective prophylactic method for cataract surgery and alone may be enough, particularly with intravitreal injections.37 Researchers have not shown that perioperative administration of topical antibiotics decreases the risk of endophthalmitis, nor does it reduce bacteria flora beyond that of 5% povidone-iodine alone.37,38 Further, recent studies suggest that serial, post-injection, topical antibiotics do not reduce endophthalmitis rates, but may actually increase antibiotic resistance.38
This has changed the practice patterns specifically for intravitreal injections in which pre-procedure povidone-iodine has been deemed sufficient.39 Meanwhile, for more invasive surgical procedures, povidone-iodine alone hasn't been deemed sufficient and alternatives to perioperative topical antibiotic drop instillation are becoming increasingly popular.39
Dropless Perioperative Prophylaxis
Some practitioners are seeking to eliminate topical antibiotic drop instillation for perioperative prophylaxis, which can drive poor patient compliance and antibiotic resistance. Interest in intracameral antibiotics for endophthalmitis prophylaxis for cataract surgery is growing. The European Society of Cataract and Refractive Surgery's study provided evidence for the use of intracameral antibiotics following cataract surgery.40 The results indicate a five-fold decrease in endophthalmitis following cataract surgery when cefuroxime was injected into the anterior chamber.40 A large-scale US study also found a significant reduction in postoperative endophthalmitis rates with the use of intracameral cefuroxime.41
Although an off-label use, this perioperative prophylactic strategy is now used by an increasing number of ophthalmologists. The challenge is that there currently isn't an FDA-approved injectable for this purpose.42 There are also safety concerns regarding dilutional and contamination errors because cefuroxime is not commercially available in a preformulated preparation and must be diluted from a powder form in the operating room.42
Intravitreal antibiotic and steroid combination use is another alternative strategy for dropless perioperative prophylaxis. Triamcinolone/moxifloxacin (Trimoxi, Imprimis Pharmaceuticals) is a compounded combination medication being used off-label in cataract and LASIK surgery. No formal large-scale randomized studies have been completed investigating this technique. However, a retrospective study of 1575 eyes injected with 15mg/1mg/ml of Trimoxi following intraocular lens implantation found it was effective in preventing inflammation, endophthalmitis and cystoid macular edema.43 In fact, there were no cases of endophthalmitis.43
Intraocular lenses soaked with antibiotics have also been examined as a means to prevent endophthalmitis. Studies show that intraocular lenses presoaked with gatifloxacin and moxifloxacin are capable of delivering clinically significant antibiotic levels in rabbits.44,45 In a recent study comparing the concentration level of intracameral injection of moxifloxacin to intraocular lenses presoaked with moxifloxacin in rabbits, intracameral antibiotic injections showed a high antibiotic concentration for a short time, but the presoaked IOLs showed slower decrease rates of the antibiotic level in the anterior chamber.46
Given these results, dropless strategies of perioperative prophylaxis show promise in the prevention of endophthalmitis, although further work is needed in this area. Protocols that include a steroid component introduce the risk of IOP elevation or transient visual blur.
Prophylactic use of antibiotics for corneal abrasions is less controversial. Despite lack of proven effectiveness for non-infected corneal abrasions, prophylactic antibiotic use persists for corneal abrasions because the compromised cornea is more susceptible to infection than an intact cornea. Broad-spectrum antibiotics are an excellent choice for corneal abrasions and other conditions for which the cornea is compromised. The preservative-free fluoroquinolones Vigamox and Moxeza (moxifloxacin 0.5%, Alcon) have broad coverage and can prevent adverse effects of preservatives on vulnerable corneas.
A Look Ahead
Despite the increase in drug resistance, antibiotic innovation has slowed over the past decade. Thankfully, there are encouraging signs of development. The primary focus has been on therapies that do not drive resistance. Instead of launching an improved antibiotic with the same target profile, the emphasis is on new antibacterial mechanisms of action and delivery methods.
Isothiazoquinolones—novel compounds in the quinolone family—show great promise as future bactericidal agents. They are structurally different from fluoroquinolones, and in addition to targeting bacterial DNA replication enzymes inhibited by fluoroquinolones, they add a distinct mechanism of action by targeting another bacterial DNA replication enzyme, DNA primase.47 Acting against the DNA primase provides a distinct mechanism of action and allows the antibiotic to act against bacteria, even in a non-dividing state.48 Additionally, research shows this added target increases antimicrobial efficacy and decreases the chance of resistance compared to fluoroquinolones.47-49 Development and commercialization of the isothiazoquinolone ACH-702 (Achillion Pharmaceuticals), an ocular antibacterial of good therapeutic potential, is underway.50
In addition to antibiotic resistance, frequent instillation of topical antibiotics, particularly for anterior segment infections, poses a significant challenge to patients. Various anterior segment antibiotic drug delivery systems are being developed to address this issue and should improve patient outcomes. Extended-release antibiotic delivery systems have the potential to maximize the therapeutic effect while minimizing resistance. Several antibiotic delivery strategies have demonstrated some success, such as hyaluron-based ocular sustained delivery of moxifloxacin; a punctal plug delivery system that elutes moxifloxacin; biodegradable implants that deliver levofloxacin; and antibiotic drug-eluting contact lenses.51-54 These ocular drug delivery systems are in early stages of development, but show promise as safe and effective techniques.
Conclusion
Bacterial infections of the anterior segment can have visually devastating consequences. Fortunately, we have a vast arsenal of antibiotics to manage these conditions and preserve the sight and quality of life of our patients. Nevertheless, the emergence of antibiotic resistance is a serious public health concern. Prudent prescribing, new antibiotics and innovative drug delivery strategies will help combat this growing issue. Antibiotics will remain our 'wonder drug' as an invaluable tool in our anterior segment disease management armamentarium.
Dr. Mickles is an associate professor at Nova Southeastern University College of Optometry and a fellow of the American Academy of Optometry.
- Schaumberg DA, Nichols JJ, Papas EB, et al. International workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011 Mar;52(4):1922-9.
- Gerd G, Tauber J, Baudoin C. The international workshop on meibomian gland dysfucntion: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):2050-64.
- John G. A comparative study in the clinical and microbial efficacy of topical besifloxacin ophthalmic suspension 0.6% with erythromycin ophthalmic ointment 0.5% for the management of acute blepharitis. Poster presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology, May 6-9 2012; Fort Lauderdale, FL.
- Duncan K, Jeng BH. Medical management of blepharitis. Curr Opin Ophthalmol. 2015;26(4):289-94.
- Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2015;32(1):44-53.6. Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19:258-63.
- Greene JB, Jeng BH, Fintelmann RE, et al. Oral azithromycin for the treatment of meibomitis. JAMA Ophthalmol. 2014;132(1):121-2.
- Kashkouli MB, Fazel AJ, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99(2):199-204.
- Liu Y, Kam WR, Ding J, Sullivan DA. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015;34(3):342-6.
- Azari A, Barney NP. Conjunctivitis a systemic review of diagnosis and treatment. JAMA. 2013 Oct 23;310(16):1721-29.
- Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: Cochrane systematic review and meta-analysis update. Br J Gen Pract. 2005;55(521):962-4.
- Fechner PU, Teichmann KD. The Conjunctiva. In: Fechner PU, Teichmann KD, eds. Ocular therapeutics. Pharmacology and clinical application. New Jersey: Slack Incorporated;1998:285-326.
- Deschênes J, Blondeau J. Besifloxacin in the management of bacterial infections of the ocular surface. Canadian Journal of Ophthalmology. 2015;50(3):184-91.
- Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145(6):951-8.
- Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J. 2009 Aug;9(2):184-95.
- Rosenberg JB, Gritz DC. Fluoroquinolones or fortified antibiotics for treating bacterial keratitis: systematic review and meta-analysis of comparative studies. Can J Ophthalmol. 2011;47(6):493-9.
- Eiferman RA, O'Neill KP, Morrison NA. Methicillin-resistant staphylococcus aureus corneal ulcers. Ann Ophthalmol. 1991 Nov;23(11):414-5.
- Dijkmans AC, Wilms EB, Kamerling IM, et al. Colistin: revival of an old polymyxin antibiotic. Ther Drug Monit. 2015 Aug;37(4):419-27.
- Catchpole CR, Andrews JM, Brenwald N, et al. A reassessment of the in-vitro activity of colistin sulphomethate sodium. J Antimicrob Chemother. 1997;39:255-60.
- Reina R, Estenssoro E, Sáenz G, et al. Safety and efficacy of colistin in acinetobacter and pseudomonas infections: a prospective cohort study. Intensive Care Med. 2005;31:1058-65.
- Michalopoulos AS, Tsiodras S, Rellos K, et al. Colistin treatment in patients with ICU-acquired infections caused by multi-resistant gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11:115-21.
- Jain R, Murthy SI, Motukupally SR, Jain M. Use of topical colistin in multiple drug-resistant pseudomonas aeruginosa bacterial keratitis. Cornea. 2014 Sept;33(9):923-7.
- Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377-82.
- Chan E, Snibson GR, Sullivan L. Treatment of infectious keratitis with riboflavin and ultraviolet-A irradiation. J Cataract Refract Surg. 2014 Nov;40:(11):1919-25.
- Schier A, Greebal G, Attia H, et al. In vitro antimicrobial efficacy of riboflavin and ultraviolet light on staphylococcus aureus, methicillin-resistant staphylococcus aureus, and pseudomonas aeruginosa. J Refract Surg. 2009 Sep;25(9):799-802.
- Tabibian D, Richoz O, Hafezi F. PACK-CXL: Corneal cross-linking for treatment of infectious keratitis. J Ophthalmic Vis Res. 2015 Jan-Mar;10(1):77-80.
- Price MO, Tenkman LR, Schrier A, et al. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012 Oct;28(10):706-13.
- Wang F. UVA/riboflavin apoptosis in mouse cornea. Ophthalmologica. 2008;222:369-72.
- Spoerl E, Wollensak G, Seller T. Increased resistance of the cross-linked cornea to enzyme degradation. Curr Eye Res. 2004;29:35-40.
- Khan YA, Kashiwabuchi RT, Martins SA, et al. Riboflavin and ultraviolet light a therapy as an adjuvant treatment for medically refractive Acanthamoeba keratitis: report of 3 cases. Ophthalmology. 2011;118:321-31.
- Makdoumi K, Mortensen J, Sorkhabi O, et al. UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2012;250:95-102.
- Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385-9.
- Khan YA, Kashiwabuchi RT, et al. Riboflavin and ultra-violet light A therapy as an adjuvant treatment for medically refractive Acanthamoeba keratitis; report of 3 cases. Ophthalmology. 2011;118:321-31.
- Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol. 2014;157(2):327-33.
- Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: The Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143-50.
- McClintic SM, Prajna NV, Srinivasan M, et al. Visual outcomes in treated bacterial keratitis: four years of prospective follow-up. Invest Ophthalmol Vis Sci. 2014 May;55(5):2935-40.
- Moss JM, Nguyen D, Liu YI, et al. Comparison of one-day versus one hour application of topical gatifloxacin in eliminating conjunctival bacterial flora. Ophthalmology. 2008 Nov;115(11):2013-6.
- Ta CN, Egbert PR, Singh K, et al. Prospective randomized comparison of 3-day versus 1 hour preoperative ofloxacin prophylaxis for cataract surgery. Ophthalmology. 2002 Nov;109(11):2036-40.
- Halachmi-Eyal O, Lang Y, Keness Y, Miron D. Perioperative topical moxifloxacin 0.55 and povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg. 2009 Dec;35(12):2109-14.
- Hsu J, Gerstenblith AT, Garg SJ, Vander JF. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without post-injection topical antibiotics. Am J Ophthalmol. 2014 Mar;157(3):514-8.
- Auletto F. Where do we stand in the battle against resistant bacteria. Review of Cornea & Contact Lenses. 2013 Nov. Accessed December 15, 2015. www.reviewofcontactlenses.com/content/d/disease/c/44959/.
- ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007 Jun;33(6):978-88.
- Shorstein NH, Winthrop KL, Kerinnton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a northern California eye department. J Cataract Refract Surg. 2013 Jan;39(1):8-14.
- Montan PG, Wejde G, Setterquist H, et al. Prophylactic intracameral cefuroxime. Evaluation of safety and kinetics in cataract surgery. J Cataract Refract Surg. 2002 Jun;28(6):982-987.
- Galloway MS. Intravitreal placement of antibiotic/steroid as substitute for postoperative drops after cataract surgery. The Annual ASCRS ASOA Symposium and Congress, April 25-29, 2014. Abstract.
- Shaw J, Smith EF, Desai RU, et al. Can intraocular lenses deliver antibiotics intracamerally? J Ocul Pharmacol Ther. 2010 Dec;26(6):587-9.
- Kleinmman G, Apple DJ, Chew J, et al. Hydrophilic acrylic intraocular lens as a drug-delivery system: Pilot study. J Cataract Refract Surg. 2006 Apr;32(4):652-4.
- Liptnitzi I, Bronshtein R, Ben Eliahu S, et al. Hydrophilic acrylic intraocular lens as a drug delivery system: Influence of the presoak time and comparison to intracameral injection. J Ocul Pharmacol Ther. 2013 May;29(4):414-8.
- Shapiro A. In vitro activity of ACH-0139586, a novel isothiazoquinolone, moxifloxacin and gatifloxacin against clinical isolates, including methicillin and fluoroquinolone resistant. Invest Ophthalmol Vis Sci. 2012;53. ARVO e-abstract 6259.
- Podos SD, Thanassi JA, Leggio M, Pucci MJ. Bactericidal activity of ACH-702 against nondividing and biofilmstaphylococci. Antimicrob Agents Chemother. 2012 Jul;56(7):3812-8.
- Pucci MJ, Podos SD, Thanassi JS, et al. In vitro and in vivo profiles of ACH-702, and isothiazoloquinolone, against bacterial pathogens. Antimicrob Agents Chemother. 2011 Jun;55(6):2860-71.
- Chapin M, Tobaru A, Sandwick V. Leveraging Ex-US Deals. Review of Ophthalmology. 2014. Accessed December14, 2015. www.reviewofophthalmology.com/content/c/50833/.
- Lee HK, Rafii MJ, Mann B, et al. Hyaluron-based ocular sustained delivery of moxifloxacin. Invest Ophthalmol Vis Sci. 2014 April;55:454.
- Chee SP. Moxifloxacin punctum plug for sustained delivery. Ocul Pharmacol Ther. 2012 Aug;28(4):340-9.
- Gao P, Nie X, Zou M, et al. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot. 2011 Sep;64(9):625-34.
- Hyatt AJ, Rajan MS, Burling K, et al. Release of vancomycin and gentamicin from a contact lens versus a fibrin coating applied to a contact lens. Invest Ophthalmol Vis Sci. 2012 Apr;53(4):1946-52.
