A Systematic Approach to Solving Contact Lens Discomfort
Comfort may be the number one factor in achieving a successful, long-lasting contact lens fit. Take a stepwise approach when comfort can’t be found.
By Christopher Kuc, OD
 |
Release Date: March 15, 2019
Expiration Date: March 15, 2022
Estimated time to complete activity: 1 hour
Jointly provided by Postgraduate Institute for Medicine and RGVCE
Educational Objectives: After completing this activity, the participant should be better able to:
- Define the characteristics and implications of contact lens discomfort.
- Take a thorough history of the patient’s problem and general health status.
- Describe the modifiable and non-modifiable patient factors, as well as the ocular and external environmental factors, that may contribute to contact lens discomfort.
- Use a stepwise intervention strategy to attempt to ameliorate contact lens discomfort and to prevent decreased or discontinued contact lens wear.
Target Audience: This activity is intended for optometrists engaged in the care of patients with contact lens discomfort.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and RGVCE. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Christopher Kuc, OD, Chester County Eye Care Associates in West Chester, PA.
Credit Statement: This course is COPE approved for 1 hour of CE credit. Course ID is 61135-CL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Kuc: Nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The RGVCE planners, managers and editorial staff have nothing to disclose.
How can a patient who has been wearing an outdated lens for decades and using generic multipurpose solution have no complaints while another patient wearing the latest in daily disposable technology borders on miserable?
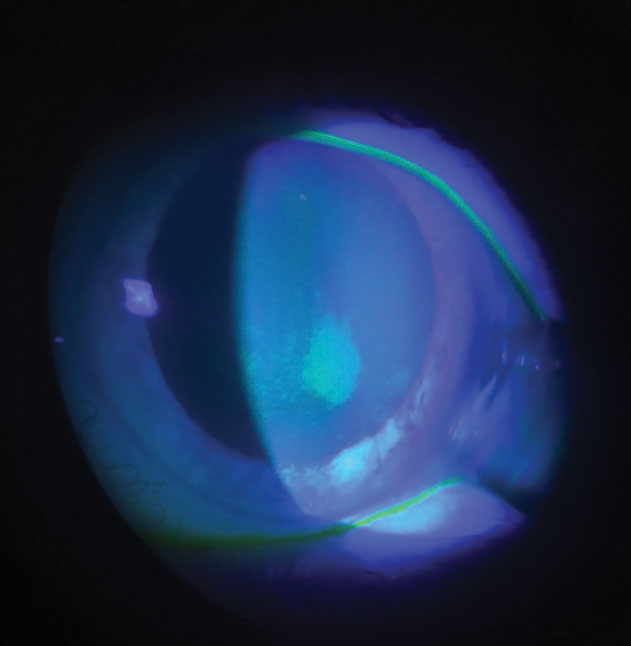 |
| Fig. 1. Staining associated with preservative uptake and release. Corneal staining should be evaluated during a contact lens exam with consideration given to underlying mechanisms. Click image to enlarge. |
When selecting an appropriate lens, the biggest obstacle for any clinician to overcome is contact lens discomfort (CLD). Surveys show this is the predominant complication for upwards of 20% of patients who drop out of contact lens wear.1,2 Indeed, as many as 50% of patients who stop wearing contacts cite CLD as their primary reason for throwing in the towel, and this dropout rate is a limiting factor in growing a contact lens practice.2,3
This article explains recent findings related to CLD and provides a stepwise approach to troubleshooting CLD in our patients.
Defining and Identifying The problem
In 2013, a group of experts known as the Tear Film & Ocular Surface Society (TFOS) published a comprehensive report on CLD. The TFOS workshop defined CLD as:
“A condition characterized by episodic or persistent adverse ocular sensations related to lens wear, either with or without visual disturbance, resulting from reduced compatibility between the contact lens and the ocular environment, which can lead to decreased wearing time and discontinuation of contact lens wear.”4
Because we want to avoid decreased wearing time and discontinuation of contact lens wear in our patients, we need to seriously address CLD.
But detecting the early symptoms of CLD can be challenging. The prevalence of discomfort and dryness revealed in contact lens surveys demonstrates that most patients will not voice their symptoms during an exam unless the clinician takes a proactive approach.
Eliciting Symptoms
One method for eliciting a patient’s symptoms is questionnaires. Implementation of a questionnaire can seem daunting, but in many cases could help grow a dry eye and contact lens practice. Commonly used validated questionnaires are the Ocular Surface Disease Index (OSDI) or the Contact Lens Dry Eye Questionnaire (CLDEQ).5 A shorter version of the CLDEQ, known as the CLDEQ-8, accurately reflects changes in patient symptoms in a simpler format.5
Taking a more thorough history can also yield symptoms that a patient may not normally discuss. Ask all contact lens patients direct questions, such as:
- Does your vision change throughout the day?
- Do your eyes become tired in the afternoon?
- Have your eyes ever become red while wearing contacts?
- How long do you use a computer or screen each day?
- Are you using lubricating drops?
- How many hours per day do you wear your contacts? Overnight?
- Can you describe how your contacts feel throughout the day?
Using at least one open-ended question to elicit symptoms is important. If you rely on technical staff for workups, then consider using a standard checklist to elicit history in your contact lens patients.
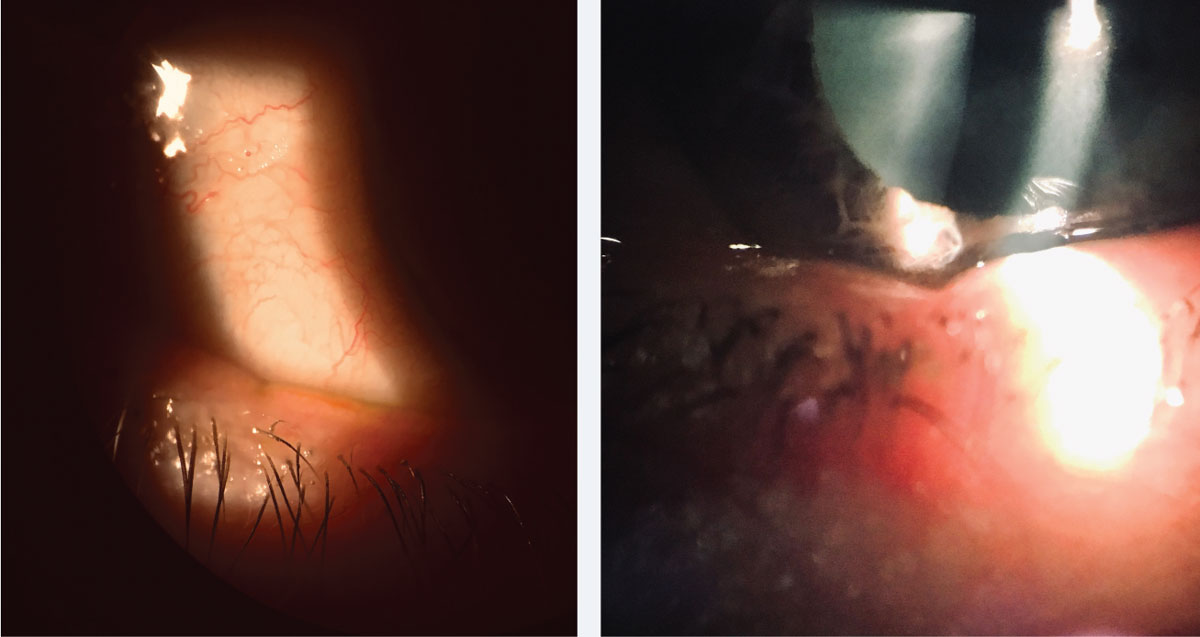 |
| Fig. 2. Lid notching indicating MGD is easily overlooked and can be a clue to underlying deficient lipid tear film layer. Click image to enlarge. |
A good tie-in to history taking is to use flow charts to help categorize contact lens discomfort. This requires little paperwork, and you gather the information simply by learning the correct questions to ask and collecting scores based on responses. One such flow chart is the Berkeley Dry Eye Flow Chart (DEFC). Research has shown a strong correlation between DEFC and leading contact lens questionnaires such as OSDI.6
Influences in CLD
Collecting a detailed history gives us information about non-modifiable and modifiable influences in contact lens discomfort.
Non-modifiable factors such as increased age and female sex, for instance, play a role in contact lens discomfort risk. Also, a history of underlying allergies, autoimmune disease and underlying disease such as polycystic ovarian syndrome have all been tied to CLD.7
Modifiable factors, in turn, can be evaluated and managed based on information gathered in a patient’s medical history. For example, the use of oral contraceptives and over-the-counter pain medications have been tied to contact lens discomfort. Modifiable environmental considerations that affect CLD include humidity, airflow and changes in blink rate with prolonged screen time.7,8
Gathering this pertinent information should be the starting point of an investigation into contact lens discomfort.
Lens Characteristics
Once you’ve identified a patient with CLD and you’ve determined patient considerations based on history, a systematic approach to isolating other contributors is important. The contact lens itself is where most practitioners would begin their focus. With the resurgence of silicone hydrogel (SiHy) lenses and benefits of increased Dk, many doctors still believe that more oxygen equals more comfort.
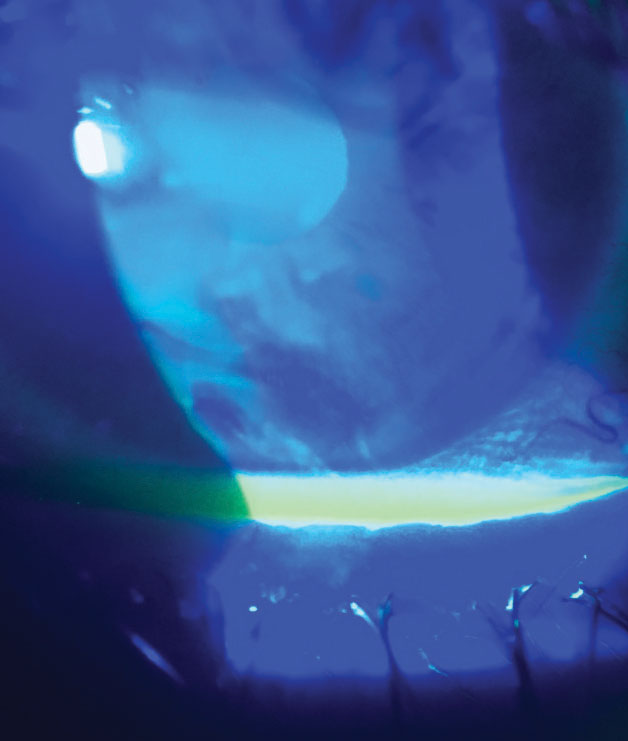 |
| Fig. 3. Reduction in tear break-up time is easily assessed and has direct correlation to CLD. Click image to enlarge. |
However, studies with good controls have not shown superior comfort with SiHy and have revealed the difficulty in finding what contributes to CLD, including lens properties, design and modality.9 The modulus of the lens, which is also closely tied to Dk, affects the rigidity or flexibility of a lens because it’s directly tied to water content. The higher the silicone content, the more challenging it is to incorporate greater water content, and the lens modulus becomes higher/stiffer. Intuitively, a stiff modulus would seem like a logical reason for an uncomfortable lens, but this too has not been verified in studies.10 Surprisingly, the TFOS workshop also found no direct correlation in CLD to deposits, dehydration and tear exchange.11
What are some modifiable contact lens properties that do have a direct correlation to CLD?
The fit of the lens is important. A lens that moves less and closely conforms to the bulbar conjunctiva tends to be more comfortable.12 This may also tie closely to lens edge profile and thickness. A thinner lens edge provides better comfort, possibly because it causes less disruption of the surrounding conjunctiva.13
A more recent area of interest is lubricity and friction. Studies indicate these may significant impact comfort, and the combined properties of a lens may individually affect a lens’s coefficient of friction.14
Other properties the TFOS identified as contributing to improved CLD are shorter frequency of replacement and lower water content.11 Overwear of contact lenses, including overnight or prolonged daily wear hours, has been difficult to study but has long been tied to contact lens discomfort and should be addressed with your patients. Discuss the importance of adhering to the manufacturer-recommended replacement schedule and duration of daily wear time.15,16
Contact Lens Solutions
Although the current eye care landscape has seen huge growth in the daily disposable market, understanding the ingredients in solutions—and how certain lens-solution combinations have been shown to contribute to CLD—is incumbent upon all optometrists who fit daily wear contacts. Because the various components in multipurpose solutions include biocides, surfactants, wetting agents, chelating agents and buffering agents, studying which elements affect contact lens discomfort has been challenging.
A good starting point for differentiating these products is understanding that the active ingredient in contact lens solutions is the biocide or preservative. Polyhexamethylene biguanide (PHMB), polyquaternium-1 (Polyquad), alexidine dihydrochloride, and hydrogen peroxide are common biocides used in contemporary solutions.
 |
| Click chart to enlarge. |
Preservative uptake and release have been extensively investigated and found to be associated with increased corneal staining (Figure 1).17,18 Although many studies showed PHMB is a greater concern for this phenomenon, the related corneal staining wasn’t closely correlated to CLD. Nevertheless, one way to minimize corneal staining is to use caution when mixing groups II, IV and silicone hydrogel materials with older PHMB.19,20
Meanwhile, hydrogen peroxide systems are often touted as providing superior comfort, and at least one smaller study has shown improved comfort and extended wearing times.21
Among newer generation solutions (including hydrogen peroxide), one component tied directly to better comfort is the wetting agent.22,23 This supports the idea that increased lubricity or decreased friction may add to comfort and should be considered when recommending solutions for silicone hydrogel lenses.
Proper use of any system, including case care, should be reviewed at each visit, as compliance is tied to keratitis and corneal staining.24 Recommending new solutions designed to match new lenses makes sense based on these findings and should be considered when discomfort occurs.
Preexisting Ocular Conditions
If the ocular surface has underlying disease, even the latest contact lens or solution may not lead to better comfort. A thorough slit lamp exam of any patient who is currently wearing or considering contacts should give insight into existing ocular pathology that may contribute to contact lens discomfort.
For instance, meibomian gland dysfunction (MGD) and CLD are closely tied.25,26 More than 20% of patients who have pre-existing evaporative dry eye do not know they have MGD.27 A careful examination of these glands—including eversion of the lower lid, transillumination and expression of meibum—is easy to do and can help identify MGD in CLD (Figure 2).
Meibography is becoming more common in optometric practice and can help screen contact lens wearers with underlying lid disease and gland atrophy. Once you’ve identified MGD, initiate traditional therapies such as hot compresses (with improved microwaveable eye masks that incorporate silica beads to promote moisture) or in-office heat therapy such as LipiFlow (Johnson & Johnson Vision), MiBo Thermoflo (Mibo Medical), or iLux (Alcon).
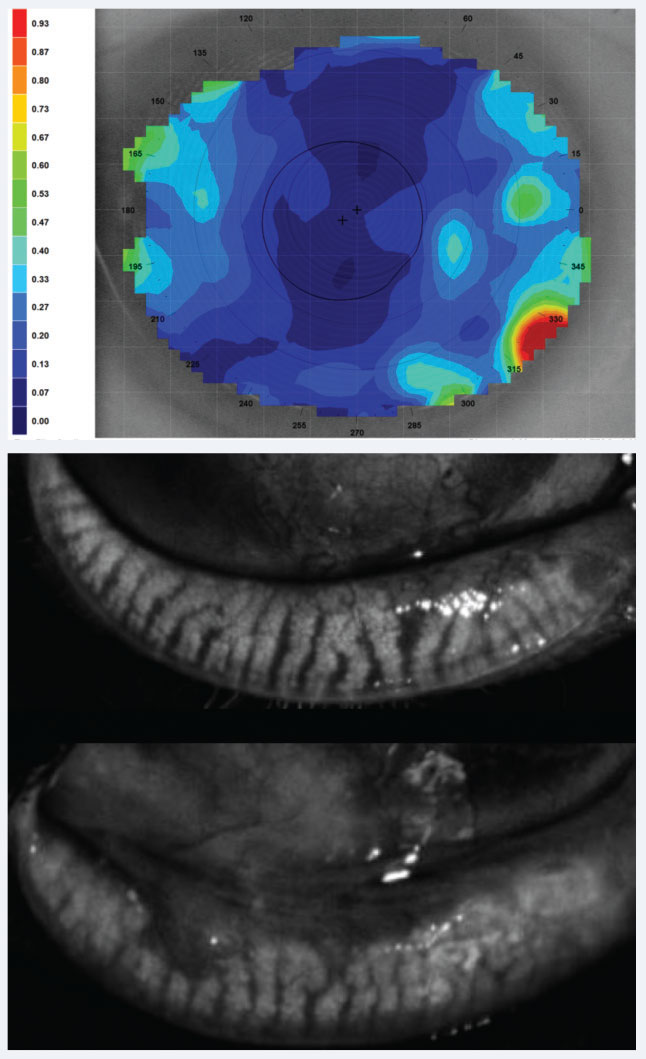 |
| Fig. 4. Above, dynamic tear film stability analysis with cooler colors representing uniform, denser tear layer and hot colors representing thinning and poor consistency, may shed light on CLD related to poor tear film quality. Below, meibography showing healthy glands above and glands with truncation and close to 50% loss inferiorly. Click image to enlarge. |
Tear film quality should also be assessed clinically. In 2017, TFOS released the DEWS II report, which again identified inflammation as playing a central role in dry eye.28 We know that contact lens wear can induce inflammation, but it has been more difficult to affix CLD to inflammation.29,30 One recent study has shown upregulation of IL-17A in tear film analysis of patients with contact lens discomfort.31 Identifying underlying inflammation should be a top priority when addressing CLD, and methods for measuring this include tear film osmolarity (TearLab) and InflammaDry (Quidel).
Also, consider adding topical steroids, cyclosporines or other anti-inflammatory agents as a possible solution to CLD if switching contacts and solutions have failed.
Tear break-up time (TBUT) is an important indicator of dry eye, which in normal eyes should be greater than 10 seconds, and can be assessed simply with fluorescein (Figure 3). In an eye wearing a contact lens, this time could be regularly diminished to eight seconds and even lower if CLD is present.32 In-office tear film testing, such as tear film stability (Medmont topographer/Oculus Keratograph) and tear film interferometry (LipiView II, Johnson & Johnson Vision), can be incorporated and is closely tied with MGD discussed earlier (Figure 4).33,34 In confounding cases, consider use of all vital dyes (lissamine green and rose bengal), as one may reveal nuanced staining patterns where another may not, such as in conjunctival staining.
Inserting silicone punctal plugs has also been demonstrated to improve CLD and can certainly be considered after first treating underlying surface inflammation, even though this avenue is traditionally used for dry eye therapy.35
To enhance tear film stability, direct the patient to use a good preservative-free surface lubricant (or one that is approved for contact lens use) before and after contact lens insertion.36
In addition, absence of sufficient surface lubrication and the presence of underlying inflammation may contribute to the development of lid wiper epitheliopathy, which is also tied to CLD.24 This abnormality to the lid margin can be identified easily with lissamine green and should also be considered in the presence of an abnormal tear film along with meibomian gland and tear film evaluation.37
In summary, CLD is an often overlooked, multifaceted condition associated with contact lens wear. Understanding its impact should encourage a practitioner to assess each contact lens patient carefully. A typical exam should consist of a thorough history (including a questionnaire when possible), evaluation of fit and proper use of solutions, consideration of lens material characteristics and biomicroscopy to detect underlying surface or adnexal disease. Implementing the knowledge available regarding CLD and taking a systematic approach to manage these patients will undoubtedly help your patients while helping your contact lens practice.
Dr. Kuc practices with the multispecialty group Chester County Eye Care Associates in West Chester, PA, where he focuses on ocular surface disease and serves as the provider liaison for practice relations. He is a fellow of the American Academy of Optometry and diplomate of the American Board of Optometry.
|
1. Pritchard N, Fonn D, Brazeau D. Discontinuation of contact lens wear: a survey. Int Contact Lens Clin. 1999;26(6):157-62. 2. Dumbleton K, Woods C, Jones L, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39(1):93-9. 3. Rumpakis J. New data on contact lens dropouts: an international perspective. Rev Optom. 2010;147(1):37-42. 4. Nichols KK, Redfern RL, Jacob JT, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS14-9. 5. Jalbert I, Golebiowski B, Stapleton F. Measuring contact lens discomfort. Curr Ophthalmol Rep. 2015;3(2)106-10. 6. Graham A, Lundgrin E, Lin M. The Berkeley Dry Eye Flow Chart: A fast, functional screening instrument for contact lens-induced dryness. PLoS ONE. 2018;13(1):e0190752. 7. Dumbleton K, Caffery B, Dogru M, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS20-36. 8. Bitton E, Jones L, Simpson T, Woods C. Influence of the blink interval on tear meniscus height in soft contact lens and nonlens wearers. Eye Contact Lens. 2010;36(3):156-63. 9. Guillon M. Are silicone hydrogel contact lenses more comfortable than hydrogel contact lenses? Eye Contact Lens. 2013;39(1):86-92. 10. Dumbleton K, Keir N, Moezzi A, et al. Objective and subjective responses in patients refitted to daily-wear silicone hydrogel contact lenses. Optom Vis Sci. 2006;83(10):758-68. 11. Jones L, Brennan N, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70. 12. Stapleton F, Tan J. Impact of contact lens material, design, and fitting on discomfort. Eye Contact Lens. 2017;43(1):32-39. 13. Maissa C, Guillon M, Garofalo RJ. Contact lens-induced circumlimbal staining in silicone hydrogel contact lenses worn on a daily wear basis. Eye Contact Lens. 2012;38(1):16-26. 14. Subbaraman L, Pruitt J, Jones L. Measuring contact lens friction. Contact Lens Spectrum. 2016;31:40-43. 15. Papas E, Tilia D, McNally J, de la Jara PL. Ocular discomfort responses after short periods of contact lens wear. Optom Vis Sci. 2015;92(6):665-70. 16. Solomon OD, Freeman MI, Boshnick EL, et al. A 3-year prospective study of the clinical performance of daily disposable contact lensescompared with frequent replacement and conventional daily wear contact lenses. CLAO J. 1996;22(4):250-7. 17. Malet F. An acute clinical comparison of corneal staining and comfort associated with contact lens care solutions. Cont Lens Anterior Eye. 2014;37(5):351-7. 18. Rosenthal RA, Dassanayake NL, Schlitzer RL, Schlech BA. Biocide uptake in contact lenses and loss of fungicidal activity during storage of contact lenses. Eye Contact Lens. 2006;32(6):262-6. 19. Jones L, Jones D. Houlford M. Clinical comparison of three polyhexanide-preserved multi-purpose contact lens solutions. Cont Lens Anterior Eye. 1997;20(1):23-30. 20. Jones L, MacDougall N, Sorbara L. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002;79(12):753-61. 21. Keir N, Woods CA, Dubleton K, Jones L. Clinical performance of different care systems with silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2010;33(4):189-95. 22. Yang SN, Tai YC, Sheedy JE, et al. Comparative effect of lens care solutions on blink rate, ocular discomfort and visual performance. Ophthalmic Physiol Opt. 2012;32(5):412-20. 23. Lemp J, Muya L, Shows A, Chen H. New peroxide lens care system demonstrates improved wetting substantivity and low residual peroxide after neutralization. Poster presented at Global Specialty Lens Symposium. Las Vegas, NV; January 21, 2016. 24. Peterson RC, Fonn D, Woods CA, Jones L. Impact of a rub and rinse on solution-induced corneal staining. Optom Vis Sci. 2010;87(12):1030-6. 25. Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc. 1980;51(3):243-51. 26. Markoulli M, Kolanu S. Contact lens wear and dry eyes: challenges and solutions. Clin Optom (Auckl). 2017 Feb 15;9:41-48. 27. Viso E, Rodríguez-Ares MT, Abelenda D, et al. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012 May 4;53(6):2601-6. 28. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 29. Efron N. Contact lens wear is intrinsically inflammatory. Clin Exp Optom. 2017;100(1):3-19. 30. Wilcox M. Is there a role for inflammation in contact lens discomfort? Eye Contact Lens. 2017;43(1):5-16. 31. Gad A, Vingrys A, Wong C, et al. Tear film inflammatory cytokine upregulation in contact lens discomfort. Ocul Surf. 2019;17(1):89-97. 32. Guillon M, Dumbleton KA, Theodoratos P, et al. Association between contact lens discomfort and pre-lens tear film kinetics. Optom Vis Sci. 2016;93(8):881-91. 33. Llorens-Quintana C, Szczesna-Iskander D, Iskander DR. Supporting dry eye diagnosis with a new method for noninvasive tear film quality assessment. Optom Vis Sci. 2019;96(2):103-10. 34. Downie, LE. Automated tear film surface quality breakup time as a novel clinical marker for tear hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2015;56(12):7260-8. 35. Giovagnoli D, Graham S. Inferior punctal occlusion with removable silicone punctal plugs in the treatment of dry-eye related contact lens discomfort. J Am Optom Assoc. 1992;63(7):481-5. 36. McDonald M, Schachet JL, Lievens CW, Kern JR. Systane Ultra lubricant eye drops for treatment of contact lens-related dryness. Eye Contact Lens. 2014;40(2):106-10. 37. Siddireddy J, Tan J, Vijay A, Wilcox M. Predictive potential of eyelids and tear film in determining symptoms in contact lens wearers. Optom Vis Sci. 2018;95(11):1035-45. |
