 |
A Stepwise Approach to Diagnosing Dry Eye Disease
Identifying this condition requires a systematic assessment using multiple techniques.
By Hamza Shah, OD, MS
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: August 15, 2024
Expiration Date: August 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists interested in effectively addressing DED and ensuring optimal outcomes.
Educational Objectives: After completing this activity, participants should be better able to:
Systematically evaluate and identify dry eye disease.
Conduct a comprehensive patient case history when DED is suspected.
Effectively use dry eye questionnaires to support the care of these patients.
Recognize when specific testing is necessary for a thorough dry eye evaluation.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Shah has no financial disclosures. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #128938 and course ID 92649-GO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
With the incidence of ocular surface disease (OSD) on the rise, evaluating the relevant structures is a crucial aspect of every eye examination. Dry eye disease (DED) ranks among the most prevalent ocular conditions worldwide, with evaporative DED being the most common form.1,2
When approaching dry eye management, it is important to keep in mind that other ocular surface diseases can influence its course. For this reason, be sure to perform a comprehensive ocular surface assessment on every patient with dry eye signs or symptoms. Without thorough assessment, misdiagnosis can lead to insufficient or misdirected treatment and persistent symptoms for the patient.
Establishing a standardized protocol for evaluating OSD patients is useful for the busy clinician. A systematic approach not only provides technicians with clear guidelines but also helps doctors avoid overlooking key details. Like glaucoma, OSD is a chronic, multifactorial condition requiring systematic assessment and ongoing treatment.
Dry eye occurs due to tear film instability, hyperosmolarity and ocular surface inflammation, resulting in damage to the ocular surface.1 Classified by the Tear Film and Ocular Surface Society’s Dry Eye Workshop (DEWS II) Definition and Classification Report, the condition can be broken down into two primary categories: aqueous-deficient dry eye and evaporative dry eye.1 When performing a dry eye workup, not only is it important to diagnose the condition when present, but it is just as important to rule out dry eye masqueraders.
 |
|
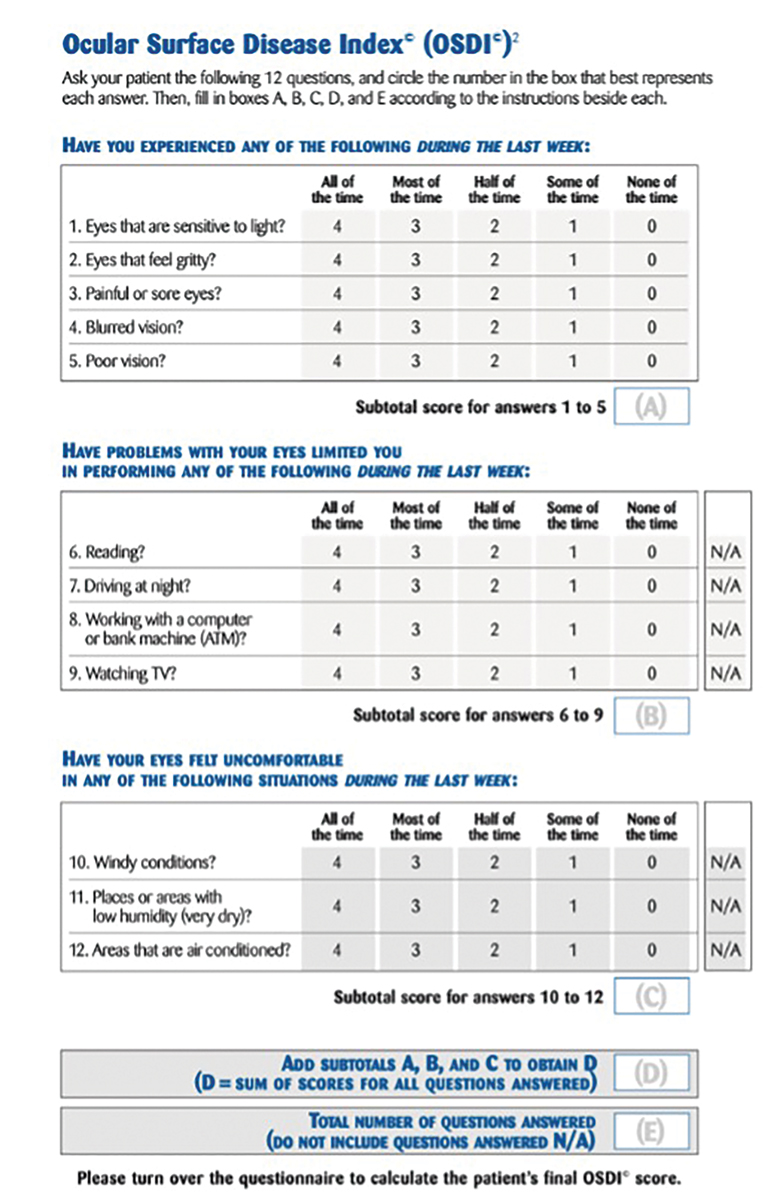
Fig. 1. Example of the OSDI questionnaire. Click image to enlarge. |
Step One: Case History
Gathering a comprehensive medical history can offer clinicians valuable insights into diagnosing and managing a patient’s DED. This includes details about their age, occupation, systemic health conditions, medications (both systemic and ocular), history of surgeries (both ophthalmic and systemic) and use of contact lenses. Symptoms of dry eye can vary widely; patients may present as asymptomatic or experience symptoms that may be constant or intermittent, such as decreased vision, irritation, redness, foreign body sensation, burning, tearing, sharp pain and photophobia.3
Systemic conditions. Many systemic conditions can contribute to DED, such as Sjögren’s syndrome, Parkinson’s disease, androgenic deficiency, thyroid disease and diabetes.4 Autoimmune conditions including rheumatoid arthritis, lupus, systemic sclerosis and dermatomyositis can lead to secondary Sjögren’s syndrome.5 Sjögren’s is a common cause for aqueous-deficient dry eye.6 It involves the secretory glands, leading to dry mouth, and can also affect the vaginal, gastric and respiratory membranes.3 Laboratory testing to consider when a clinician suspects a systemic etiology are Ro/SS-A, La/SS-B, rheumatoid factor (Rh factor), antinuclear antibodies (ANA), antibodies salivary gland protein 1 (anti-SP-1), carbonic anhydrase 6 (anti-CA6) and parotid secretory protein (anti-PSP), all of which are comprised within the Sjö test.3 In cases of high suspicion of Sjögren’s but negative blood testing, repeat blood testing or order a salivary gland biopsy.7
Medications. Another significant contributor to DED is medication use, with approximately 22 of the top 100 best-selling systemic drugs known to cause DED.8 Among these meds are antihistamines, antihypertensives, antiarrhythmics, antipsychotics, bronchodilators, antispasmodics/antimuscarinics, antineoplastics, antidepressants, antimalarials, antivirals, thiazide diuretics, cannabinoids, analgesics, chelating agents, systemic hormones, nonsteroidal anti-inflammatory, corticosteroids, anticholinergic, isotretinoin and chemotherapy agents.3,9 Additionally, topical medications containing preservatives are also recognized contributors to DED, with BAK-containing topical medications being the worst for the ocular surface.10
Other factors to consider are menopausal status, smoking status, hormone replacement therapy, vitamin A or omega-3 fatty acid deficiency and history of radiation therapy.4
 |
|
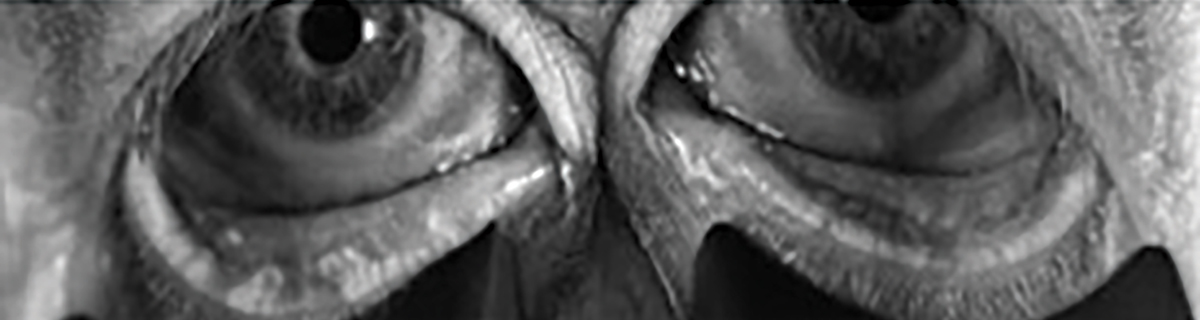
Fig. 2. Bilateral meibography with truncation and presence of tortuosity and segmentation. Click image to enlarge. |
Step Two: Dry Eye Questionnaires
There are several questionnaires available that can be used to get a subjective, quantified understanding of a patient’s symptomology before even talking with them. The questionnaires can allow clinicians to gain information regarding symptom severity, frequency, level and triggers.
Some commonly used are Ocular Surface Disease Index (OSDI), Standard Patient Evaluation of Eye Dryness (SPEED), National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25) and the Dry Eye Questionnaire-5 (DEQ-5). These questionnaires provide an unbiased number that can be used to monitor the subjective progress. Patients can sometimes be intimidated when discussing their symptoms with the doctor and can unconsciously lessen the severity of their DED.
The OSDI is a validated symptom survey often used for assessing DED severity in research (Figure 1).11 It is scored on a scale of 0 to 100, with higher scores indicating more severe cases. The questionnaire consists of three categories: ocular symptoms, visual-related function and environmental triggers. Each category comprises four questions, allowing patients to respond with a score ranging from 0 to 4 or select “N/A” for each question. The OSDI score is calculated through the following formula: (sum of score)*25/(# of questions answered). A normal OSDI score could range from 0 to 12. Dry eye is diagnosed as mild if the score is between 12 to 22, moderate if between 23 and 32 and severe if exceeding 33.12
 |
|
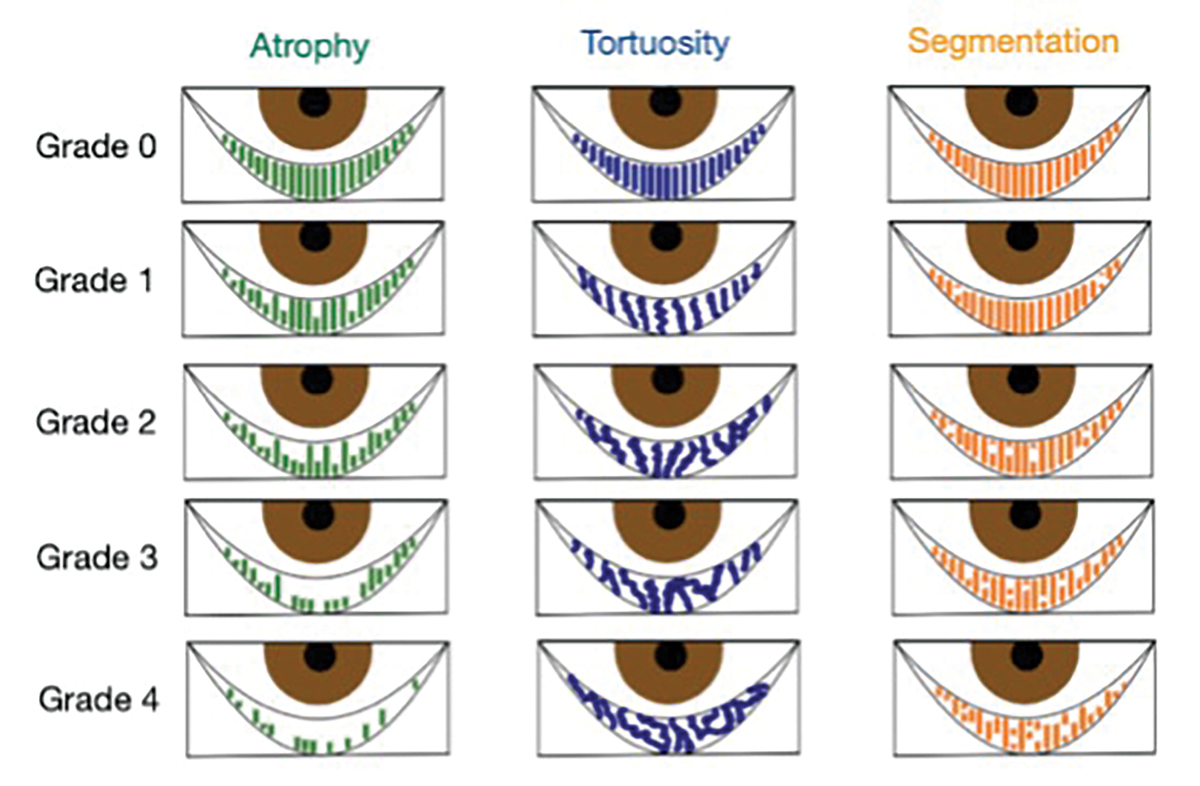
Fig. 3. Puly 5-grade scale for gland atrophy, Halleran grading scale for tortuosity and the segmentation grading scale. Diagram by Zuneera Anjum, OD. Click image to enlarge. |
Step Three: Tear Film Biomarker Assessment
Although not required for the diagnosis of DED, osmolarity and MMP-9 testing offer additional tools to help establish a diagnosis.
Tear osmolarity measurements provide a quantified assessment of solute particles within a patient’s tear film. Beyond clinical value, osmolarity findings provide a quantified tool that aids patient education. The TearLab osmometer (Bausch + Lomb), ScoutPro osmometer (Bausch + Lomb) and I-Pen osmolarity system (I-Med Pharma) are devices that measure tear osmolarity.13 TearLab analyzes a 50µL tear sample to determine tear osmolarity.14 Values of 308mOsm/L and above, or an asymmetry of at least 8mOsm/L between the eyes, are considered abnormal. Readings between 308mOsm/L and 316mOsm/L indicate mild DED, while values above 316mOsm/L indicate severe disease. Tear hyperosmolarity increases with the severity of dry eye disease.15
Hyperosmolarity in tears is due to a relative decrease in aqueous levels and an increase in solids within the tears.16 This state triggers the inflammatory cascade, leading to epithelial cell dysfunction and death, along with changes in mucin expression.1,8 Patients consequently experience chronic epithelial stress, tear instability and ocular irritation.17 It is important to consider osmolarity as a diagnostic tool among other tests, rather than the gold standard for diagnosing DED.
The DEWS II report concluded that tear osmolarity is a crucial test in diagnosing DED.1 Even though precorneal tears can be more difficult to collect, it is important to note that precorneal tear film osmolarity is significantly higher than that of the tear meniscus in DED, with values reaching as high as 800mOsm/L to 900mOsm/L.18,19 Note that one study indicated osmolarity results can sometimes be misleading, showing no significant difference between healthy and dry eye patients, and some healthy patients having higher osmolarity than the normal 308mOsm/L.20 Osmolarity can be considered an indirect test for ocular inflammation, but the results should be interpreted in conjunction with other findings.
Ideally, osmolarity should be paired with MMP-9 testing, which provides a direct indication of ocular inflammation. In-office MMP-9 rapid testing takes about 10 minutes to deliver either a negative or positive result, rather than a quantified value. The test uses a matrix metalloproteinase-9 (MMP-9) concentration of 40ng/mL as the cutoff; any concentration above this value results in a positive result, while concentrations below it yield a negative result.21
 |
|
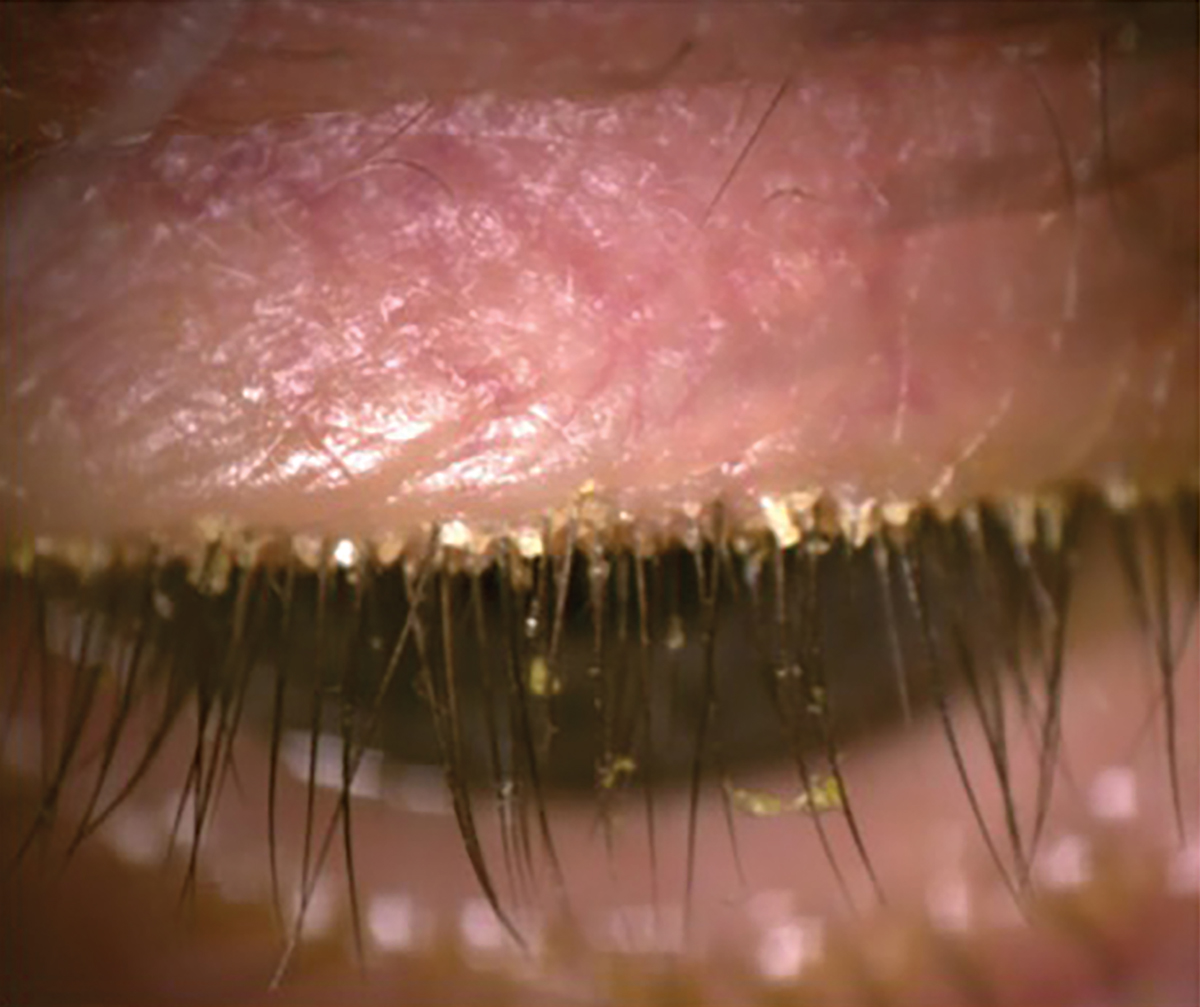
Fig. 4. Presence of Demodex with the patient looking down. Click image to enlarge. |
MMP-9 is induced by interleukins, tumor necrosis factor, tumor growth factor and the inflammatory cascade, making it an ideal marker for inflammation.22,23 A study showed that MMP-9 concentrations correlate with patients’ symptoms, tear break-up time (TBUT), corneal staining and conjunctival staining.24 Although the InflammaDry test does not provide a numerical concentration, knowing that inflammation is increased can help in formulating an accurate diagnosis and treatment plan. Positive MMP-9 results can also occur in other inflammatory conditions like allergies, chronic blepharitis and conjunctivochalasis.25,26 Therefore, it is important not to use this test as a standalone diagnostic tool, considering its positive results can be due to etiologies other than dry eye disease.
Step Four: Meibography
Although this too is not required for the diagnosis or management of DED, meibography is a great tool to assess meibomian gland quality and quantity. It also provides patients a visual representation of their gland architecture, helping them better understand their anatomy and the stage of their disease.
Various instruments facilitate meibography, including LipiView/LipiScan (Johnson & Johnson Vision), Keratograph 5M (Oculus), iLux (Alcon) and Myah (Topcon). Additionally, standalone options—anterior segment cameras with infrared capabilities—are also available to visualize the meibomian glands. Obtaining meibography images provides valuable information for the physician and serves as an excellent tool to educate patients about the condition of their glands.
When interpreting a meibography, it is important to grade the quality and quantity of the glands by analyzing three key factors: atrophy, tortuosity and segmentation, each of which can be graded from 0 to 4 (Figures 2 and 3).
Atrophy, described by the Pult 5-grade scale, measures the loss of meibomian glands, ranging from partial to complete loss from the orifice to the fornix.27 Tortuosity, assessed using the Halleran grading scale, is present if a gland bends at a 45º angle from the midline or if there are multiple bends of any degree within a gland.28 Segmentation, evaluated using a segmentation grading scale, describes the broken appearance of the glands, often indicated by a division within a gland marked by a black line.29 These grading systems provide a quick and standardized method for documenting the quality of meibomian glands as shown on the meibography.
 |
|
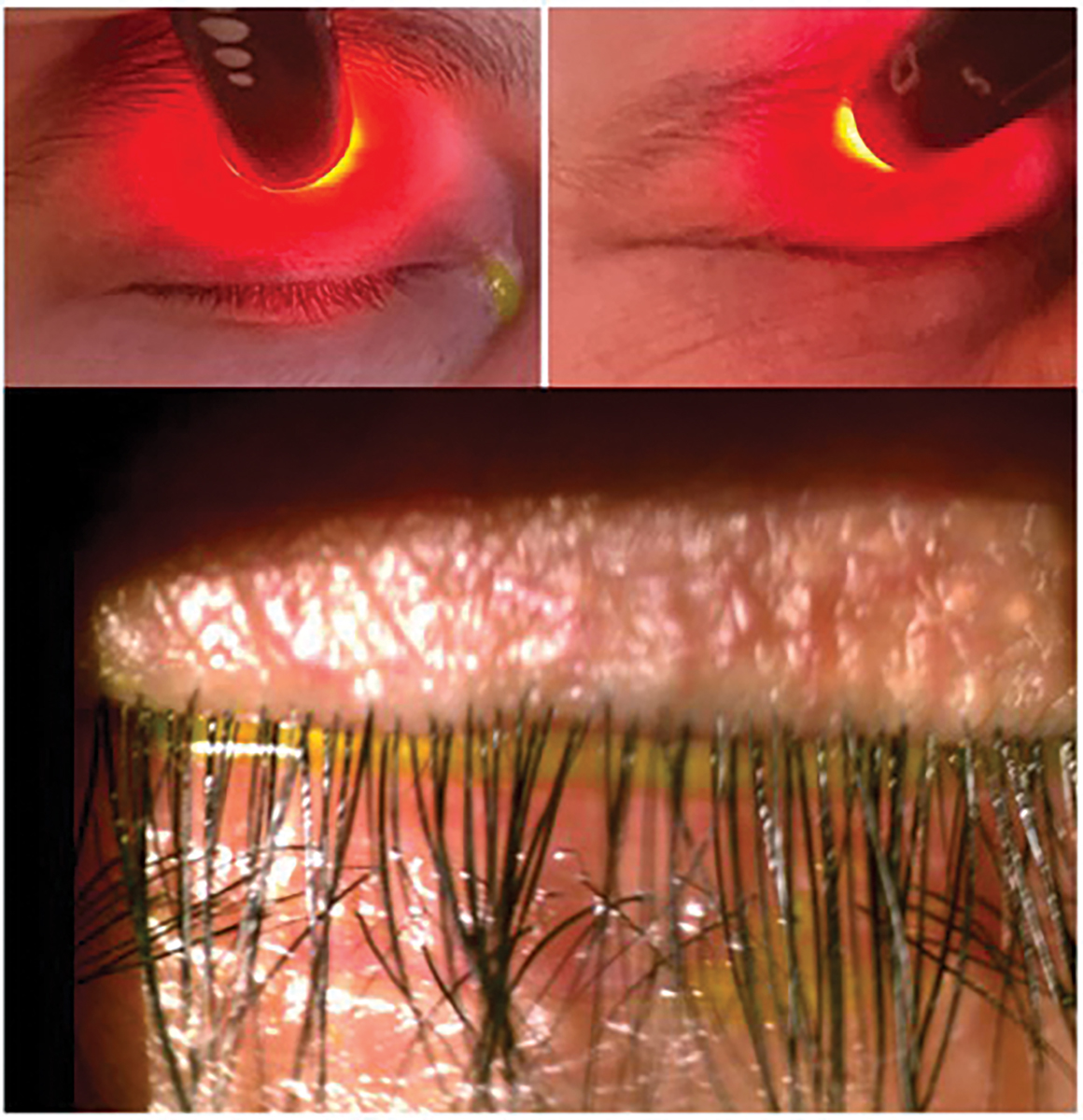
Fig. 5. Korb Blackie test reveals light leakage centrally indicating lagophthalmos (top left). Patient demonstrates no light leakage while squeezing (top right). Slit lamp photo depicting improper lid closure (bottom). Click image to enlarge. |
Step Five: Lids and Lashes
Evaluation of the eyelids and lashes should be the first step during a slit lamp examination when performing a dry eye evaluation. Information obtained from this examination can either directly contribute to diagnosing DED or help identify another condition that may be causing the patient’s symptoms. Examining the position of the lashes and lids can help rule out trichiasis, entropion and ectropion, any of which can disrupt the ocular surface. Lid abnormalities such as entropion and ectropion are more commonly seen in the elderly and are more frequently observed in female patients.30,31
Anterior blepharitis and Demodex are closely associated with meibomian gland dysfunction (MGD).32,33 Demodex is classically identified by collarettes at the base of the lashes, whereas anterior blepharitis presents with more generalized crusting and scaling along the lid margins, rather than being concentrated at the base of the eyelashes.
Anterior blepharitis. This condition is a result of overgrowth of bacteria and its associated biofilm, resulting in increased concentration of exotoxins and lipases along the lid margin.34 If not treated, this chronic inflammation leads to increased MGD, telangiectasia and hyperkeratinization.26,35,36
Demodex. One study found a 60% prevalence of Demodex infestation in the eyelids of patients with MGD, compared to 18% in controls.37 OSDI scores, corneal staining, chalazion presence, reduced TBUT and MGD are much more commonly seen in patients with Demodex.38-40 As Demodex go through their two-week life cycle, their remains contribute to the physical blockage of meibomian gland orifices and cause changes to the gland architecture over time.39,40 To obtain the best view when examining for blepharitis or Demodex, direct the patient to look down, allowing for a direct view of the base of the lashes (Figure 4). Chronic blepharitis and Demodex infestation cause chronic inflammation, leading to lid margin keratinization and, in later stages, even ectropion or entropion.26
When evaluating the lids, it is also important to check for incomplete blinking and nocturnal lagophthalmos. This can be done either with or without the slit lamp. Decreased blink frequency and incomplete blinking is increasingly seen with the rise in digital screen use.41 The average American adult spends over 10 hours per day in front of a digital screen, making it essential to look for incomplete blinking in all patients.42 Incomplete blinking has been linked to a twofold increase in the risk of developing DED and is associated with increased OSDI scores, MGD and reduced TBUT.43,44 Lagophthalmos can often be coupled with incomplete blinking. The Korb-Blackie test evaluates the level of lid closure (Figure 5). Patients with lagophthalmos have trouble keeping their eyes closed throughout their sleep, leading to increased tear evaporation. As a result, they usually report symptoms being worst upon awaking.45
 |
|
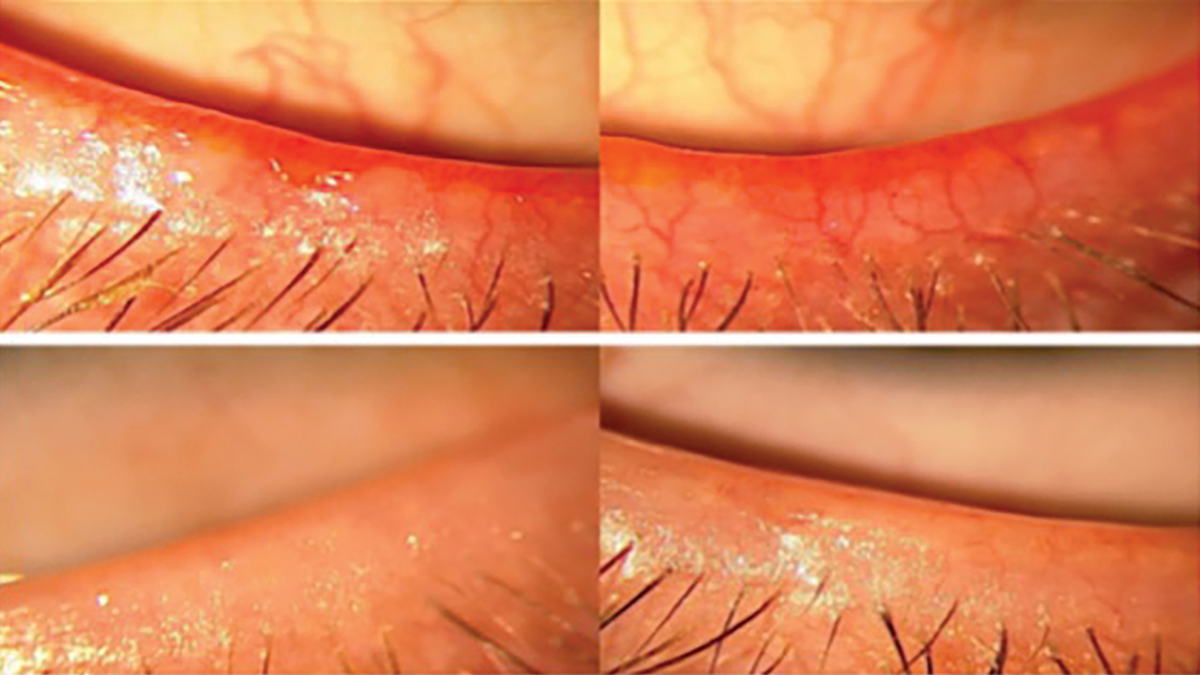
Fig. 6. Lower eyelids with telangiectasia before and after intense pulse light treatment. Click image to enlarge. |
Ocular rosacea. It is also important to notice any telangiectatic blood vessels along the lid margin (Figure 6). It is theorized that ocular rosacea is caused by increased inflammation, which triggers the expression of certain cytokines and antimicrobial molecules like cathelicidin, which has both vasoactive and proinflammatory properties. There is an increase in vascular endothelial growth factor (VEGF) within the skin, explaining the excessive vascularization seen with ocular rosacea.46,47 This can be graded from a scale of 0 to 3:48
0 = no telangiectasia
1+ = mild
2+ = moderate
3+ = severe
Step Six: Meibomian Glands
Meibum is the substance secreted by the meibomian glands. In eyes without MGD, meibum has a melting point of around 32° C.49 However, the melting point of meibum can increase up to 45° C.38,50 This phenomenon explains why in cold temperatures, such as during winter, the meibum can harden, preventing its proper release into the tear film, thereby increasing tear evaporation. MGD is a leading cause of evaporative dry eye, which is the most common form of DED.46
For a thorough evaluation of the meibomian glands, it’s essential for the physician to perform meibomian gland expression. This allows for the assessment of both the quality of meibum and the efficiency of its release (Figure 7). Meibum quality can be graded on a scale ranging from 0 to 3+. Grade 0 indicates clear meibum, considered normal, while grade 1 represents waxy or cloudy meibum with diffusely turbid fluid secretions. Grade 2 signifies granular meibum, characterized by turbid fluid secretions containing particulate matter, and Grade 3+ denotes opaque or inspissated meibum, which may have a semi-solid plug-like consistency requiring extra pressure for expression.51
When examining the glands, the presence of notching at the lid margin indicates areas of complete gland dropout.4 Additionally, meibomian foam or frothing (saponification) may be observed in patients with MGD. Frothing of the tears occurs when microbial flora produce biofilm, releasing exotoxins and lipase, leading to inflammation at the lid margin. This process breaks down tear lipids into soaps and free fatty acids, resulting in saponification of the tear film.33,52
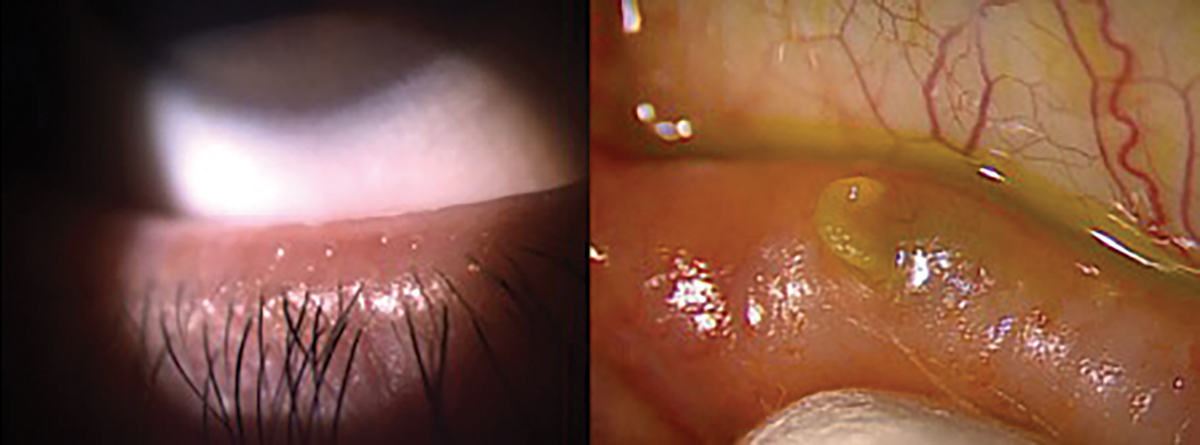 |
|
Fig. 7. Proper release of meibum through blinking, grade 0 (left). Expression of hardened meibum, grade 3+ (right). Click image to enlarge. |
Step Seven: Corneal Sensitivity
During every initial dry eye consultation, corneal sensitivity testing should be conducted before the instillation of any topical anesthetic. This test can be performed with a Cochet-Bonnet esthesiometer or with the use of a cotton tip or dental floss. It is important to assess all four peripheral quadrants (nasal, temporal, superior, inferior) and the central cornea.53
Deficient corneal sensitivity is an indicator for dysfunction of the nasociliary branch of the trigeminal cranial nerve. Early neurotrophic keratitis (NK) can present with a keratitis that looks similar to that seen with DED. Although traditional dry eye treatment does benefit patients with NK, it is not necessarily treating the root cause and, for that reason, early identification and appropriate management is necessary. NK classically presents with reduced corneal sensation and impaired corneal healing, which can lead to corneal ulceration.54
Step Eight: Tear Quality and Ocular Surface
Tear quality and ocular surface assessment is pivotal in the diagnosis of DED. Using vital dyes and conducting tests such as Schirmer and Jones can provide valuable information for an accurate diagnosis.
Tear film. Normal tear meniscus height ranges from 0.2mm to 0.5mm.55,56 A meniscus of less than 0.2mm can indicate aqueous-deficient DED.57 If you don’t wish to quantify the tear meniscus height, it is a good habit to grade it as low, average or high when viewed under the slit lamp (Figure 8).
Schirmer 1 test, without anesthetic, is another method to obtain a quantitative value of tear production. A Schirmer strip is placed into the lower fornix for five minutes, measuring both basal and reflex tearing. Schirmer 1 can also be conducted with the addition of an anesthetic drop, if trying to measure just basal tear volume. A measurement of less than 10mm wetting is considered abnormal.46
 |
|
Fig. 8. Low tear meniscus. Click image to enlarge. |
Dry eye patients may experience epiphora, but it’s crucial to rule out other causes.58 Ensure the puncta are patent, the lid architecture is intact without ectropion, and there is no blockage within the lacrimal system. Jones 1 and 2 tests are invaluable for assessing suspected lacrimal system obstructions.59 In the Jones 1 test, fluorescein dye is instilled in the fornix; if dye is present upon the patient blowing their nose, it indicates a positive result. If Jones 1 is negative, Jones 2 can be performed by dilating the puncta and irrigating the lacrimal system with a cannula. Sensation or the taste of saline confirms the patency of the lacrimal system.60
Ocular surface. Vital dye staining is a crucial component of a dry eye assessment. Several dyes can be used for evaluating the ocular surface, including sodium fluorescein (NaFl), lissamine green and rose bengal.
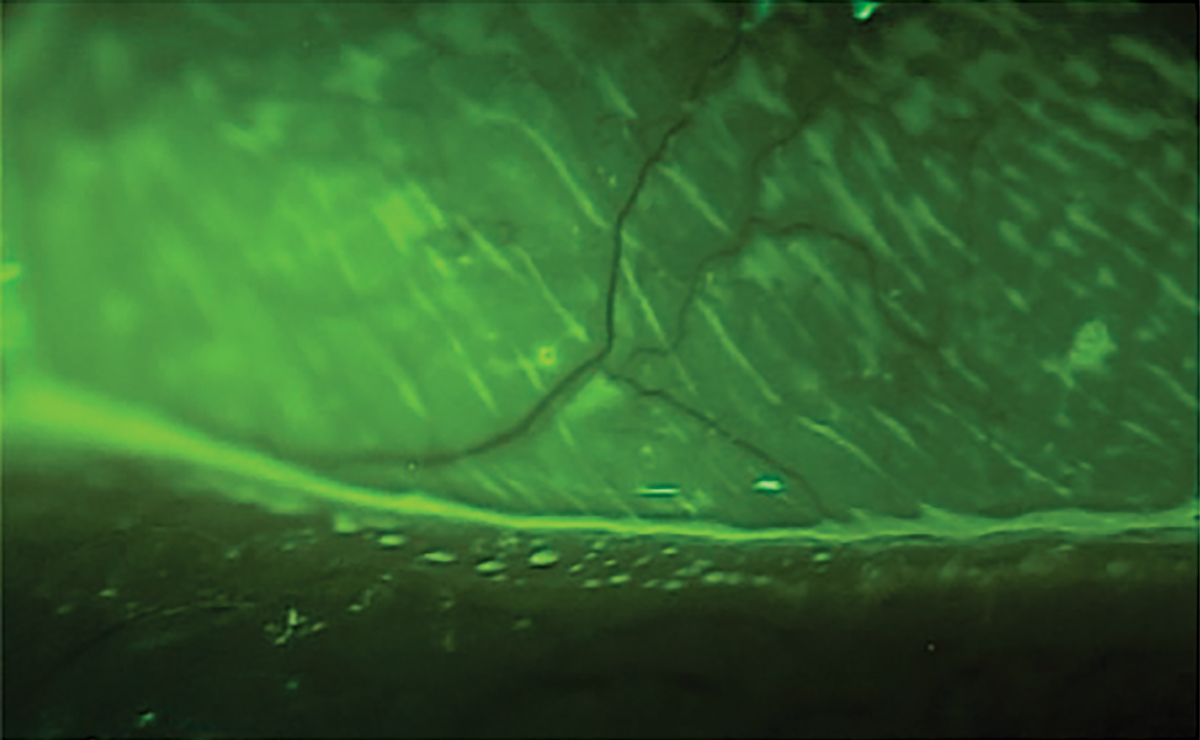
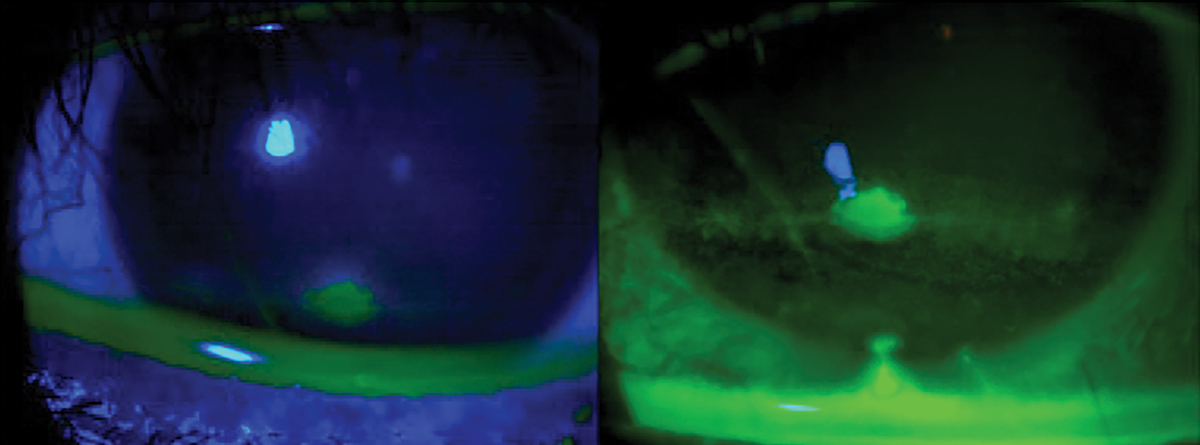
The standard dye for ophthalmic assessment is NaFl.2 It is important to use a very small amount to avoid excess dye and improper assessment. Avoid the use of a combined sodium fluorescein and anesthetic drop due to its anesthetic properties so the assessment is not affected. Also, avoid the introduction of excess dye into the tear film. NaFl theoretically can stain normal healthy cells but has a much higher affinity for areas where there is a disruption of the cell-to-cell junctions.2,50 The staining is best appreciated with a Wratten filter in conjunction with cobalt blue light, and conjunctival staining can also be better appreciated with the filter (Figure 9). NaFl can also be used to calculate the TBUT, which allows for the assessment of tear stability and evaporative DED. A TBUT greater than 10 seconds is considered normal, less than five seconds is low and anything in between is marginal.61
 |
|
Fig. 9. Ocular surface viewing with just cobalt blue light (left) with a Wratten filter. Click image to enlarge. |
Lissamine green and rose bengal are two other, less-used vital dyes for ophthalmic staining assessment. Lissamine green stains epithelial corneal and conjunctival cells that are unprotected by mucin or glycocalyx, along with degenerated and dead cells.62 Due to its better contrast than NaFl, lissamine green is excellent for assessing conjunctival staining and for lid wiper epitheliopathy without the need for a Wratten filter. However, a study concluded NaFl staining with a Wratten filter to be more sensitive in detecting conjunctival staining than lissamine green, while also allowing for corneal assessment.63 Rose bengal is less commonly used due to its toxicity and stinging upon instillation. Similar to lissamine green, it stains degenerated and dead cells.64 There have been some findings indicating that rose bengal, along with NaFl, can also stain normal healthy epithelial cells.65
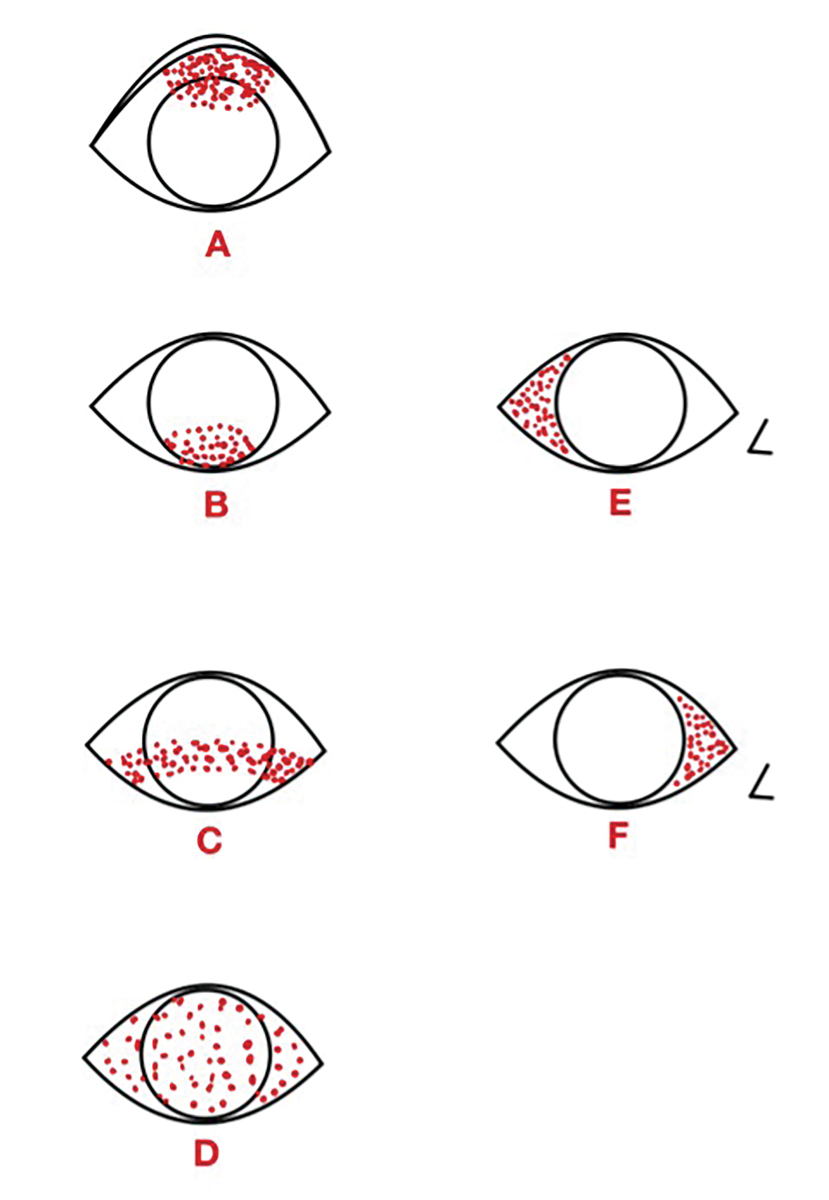
Understanding staining patterns can often help narrow the differential diagnoses (Figure 10).3,66 Superior corneal and/or conjunctival staining, as shown in Figure 10A, can be due to superior limbic keratoconjunctivitis, trichiasis or a foreign body. Figure 10B illustrates inferior staining, which can be due to lagophthalmos or incomplete blinking. An inferior-central band, depicted in Figure 10C, can result from exposure or NK. Diffuse staining, shown in Figure 10D, can indicate viral conjunctivitis or a toxic reaction.3 Temporal conjunctival staining, as seen in Figure 10E, is often associated with Sjögren’s syndrome.67 Lastly, Figure 10F shows nasal conjunctival staining, which can be one of the first areas affected in DED patients.64
 |
|
Fig. 10. Common ocular surface staining patterns. Diagram illustrated by Zuneera Anjum, OD. Click image to enlarge. |
While assessing the cornea and conjunctiva for staining, it is important to look for conjunctivochalasis (CCH). This condition, which is the result of loss of elasticity within the conjunctiva, most commonly affects the inferotemporal bulbar conjunctiva.68 Patients with CCH can present with symptoms similar to DED, which worsen in downgaze. These patients also tend to experience blurry vision and discomfort that does not resolve with blinking. This is because the upper eyelid, instead of collecting tears, contacts the loose conjunctiva and is unable to spread the tears onto the corneal surface properly.69
Final Remarks & Discussion
The follow-up schedule is as important as the initial workup and prescribed treatment for managing DED. Given that dry eye is a chronic condition, it should be treated with the same long-term care approach as other chronic ocular conditions. Patients should be seen at appropriate intervals based on the severity of their condition. Regular follow-ups and repeat diagnostic testing are essential to ensure that DED does not worsen and to catch any progression early, allowing for timely management.
The most essential tools for managing dry eye include a slit-lamp, sodium fluorescein strips and a method for capturing slit-lamp photos. It is important to document photos for comparison and even more important for patient education.
The prevalence of DED necessities a mode for its assessment during every comprehensive exam. It should be managed and treated like any other serious ocular condition. Clinicians now have a wide array of treatment options, and more are on the horizon. An accurate diagnosis is essential before initiation of treatment, given addressing the underline condition rather than just the symptoms will yield the best outcome for the patient. By establishing a stepwise approach, clinicians can effectively address DED, ensuring optimal outcomes and improved quality of life for patients.
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 2. Wróbel-Dudzińska D, Osial N, Stępień PW, et al. Prevalence of dry eye symptoms and associated risk factors among university students in Poland. Int J Environ Res Public Health. 2023;20(2):1313. 3. Wahyu T. Examination for dry eyes: dry eye syndrome. In: Modern Diagnostic Techniques and Advanced Treatments. Ferreri FM, ed. IntechOpen. 2022. 4. Tong L, Lim L, Tan D, et al. Assessment and Management of Dry Eye Disease and Meibomian Gland Dysfunction: Providing a Singapore Framework. Asia Pac J Ophthalmol (Phila). 2021;10(6):530-41. 5. Golden MI, Meyer JJ, Patel BC. Dry eye syndrome. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK470411/#. Last updated April 3, 2023. Accessed July 15, 2024. 6. Liew MS, Zhang M, Kim E, et al. Prevalence and predictors of Sjögren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96(12):1498-1503. 7. Liao R, Yang HT, Li H, et al. Recent advances of salivary gland biopsy in sjögren’s syndrome. Front Med (Lausanne). 2022;8:792593. 8. PDR Staff. Physicians’ Desk Reference. 64th ed. PDR Network; 2010. 9. Fraunfelder FT, Fraunfelder FW, Chambers WA. Clinical ocular toxicology. Elsevier Saunders; 2008. 10. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2011;153(1):1-9 e2. 11. Cartes C, Segovia C, Calonge M, et al. International survey on dry eye diagnosis by experts. Heliyon. 2023;9(6):e16995. 12. Grubbs JR, Tolleson-Rinehart S, Huynh K, et al. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33(2):215-18. 13. Messmer EM, Schaumberger MM, Priglinger S, et al. Evaluation of tear film osmolarity using Tearlab and I-Pen osmometry. Invest Ophthalmol Vis Sci. 2019;60(9):6773. 14. Sullivan BD, Whitmer D, Nichols KK. An objective approach to severity in dry eye disease. Invest Ophthalmol Vis Sci. 2010;51(12):6125-30. 15. Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35(7):553-64. 16. Tomlinson A, Madden LC, Simmons PA. Effectiveness of dry eye therapy under conditions of environmental stress. Curr Eye Res. 2013;38(2):229-36. 17. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50(8):3671-9. 18. Messmer EM, Bulgen M, Kampik A. In: Research Projects in Dry Eye Syndrome; Vol. 45:129-38 (Karger Publishers, 2010). 19. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50(8):3671-9. 20. Tashbayev B, Utheim TP, Utheim ØA, et al. Utility of tear osmolarity measurement in diagnosis of dry eye disease. Sci Rep. 2020;10(1):5542. 21. Sambursky R, Davitt WF, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24-8. 22. Corrales RM, Stern ME, De Paiva CS, et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(8):3293-302. 23. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526-35. 24. Chotikavanich S, de Paiva C, Quan Li D, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:7:3203. 25. Chhadva P, Alexander A, McClellan AL, et al. The impact of conjunctivochalasis on dry eye symptoms and signs. Invest Ophthalmol Vis Sci. 2015;56(5):2867-71. 26. Bore M. Managing ocular allergy in resource-poor settings. Community Eye Health. 2016;29(95):47-9. 27. Pult H, Riede-Pult BH. Non-contact meibography: keep it simple but effective. Cont Lens Anterior Eye. 2012;35(2):77-80. 28. Halleran C, Kwan J, Hom M, et al. Agreement in reading centre grading of meibomian gland tortuosity and atrophy. Poster at AAO Congress 2016. 29. O’Dell L, Halleran C, Schwartz S, et al. An assessment of subjective meibography image grading between observers and the impact formal gland interpretation training on inter-observer agreement of grading scores. Invest Ophthalmol Vis Sci. 2020;61(6):486. 30. Hintschich C. Correction of entropion and ectropion. Dev Ophthalmol. 2008;41:85-102. 31. Damasceno R, Osaki M, Dantas P, et al. Involutional entropion and ectropion of the lower eyelid: prevalence and associated risk factors in the elderly population. Ophthalmic Plast Reconstr Surg. 2011;27(5):317-20. 32. Rynerson JM, Perry HD. DEBS—a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10:2455-67. 33. Liang L, Ding X, Tseng S. High prevalence of Demodex brevis infestation in chalazia. Am J Ophthalmol. 2014;157(2):342-8.e1. 34. Pflugfelder S, Bauerman R, Stern ME. Dry eye and ocular surface disorders. 1st ed. CRC Press; 2004:255-7. 35. Eberhardt M, Rammohan G. Blepharitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK459305/#. Last updated January 23, 2023. Accessed July 15, 2024. 36. Mathers WD. Meibomian gland disease. In: Pflugfelder SC, Beuerman RW, Stern ME. Dry eye and ocular surface disorders. Marcel Dekker; 2004:253,260. 37. Bhandari V, Reddy JK. Blepharitis: always remember Demodex. Middle East Afr J Ophthalmol. 2014;21(4):317-20. 38. Hao Y, Zhang X, Bao J, et al. Demodex folliculorum infestation in meibomian gland dysfunction related dry eye patients. Front Med (Lausanne). 2022;9:833778. 39. Zhang AC, Muntz A, Wang MTM, et al. Ocular Demodex: a systematic review of the clinical literature. Ophthalmic Physiol Opt. 2020;40(4):389-432. 40. Spickett SG. Studies on Demodex folliculorum Simon. Parasitology. 1961;51:181-92. 41. Cruz AAV, Garcia DM, Pinto CT, et al. Spontaneous eyeblink activity. Ocul Surf. 2011;9(1):29-41. 42. Nielsen. The Nielsen total audience report: August 2020. www.nielsen.com/insights/2020/the-nielsen-total-audience-report-august-2020/. Accessed May 20, 2024. 43. Wang MTM, Tien L, Han A, et al. Impact of blinking on ocular surface and tear film parameters. Ocul Surf. 2018;16(4):424-9. 44. Jie Y, Sella R, Feng J, et al. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf. 2019;17(3):440-6. 45. Blackie CA, Korb DR. A novel lid seal evaluation: the Korb-Blackie light test. Eye Contact Lens. 2015;41(2):98-100. 46. Del Rosso JQ. Advances in understanding and managing rosacea: Part 1. Connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5(3):16-25. 47. Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55(2):77-81. 48. Arita R, Minoura I, Morishige N, et al. Development of definitive and reliable grading scales for meibomian gland dysfunction. Am J Ophthalmol. 2016;169:125-37. 49. Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17(2):360-4. 50. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-64. 51. Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond). 1991;5( Pt 4):395-411. 52. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805-17. 53. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107-31. 54. Semeraro F, Forbice E, Romano V, et al. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191-7. 55. Kulkarni SP, Bhanumathi B, Thoppil SO, et al. Dry eye syndrome. Drug Dev Ind Pharm. 1997;23:465-71. 56. Marquardt R. Diagnostische tests zur beurteilung des tränenfilms. Klin Monatsbl Augenheilkd. 1986;189(2):87-91. 57. Findlay Q, Reid K. Dry eye disease: when to treat and when to refer. Aust Prescr. 2018;41(5):160-3. 58. Ceylanoglu KS, Acar A, Sen E. Overview of epiphora referred to oculoplastic surgery clinic in adults. Beyoglu Eye J. 2023;8(1):45-9. 59. Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther. 2020;9(4):877-88. 60. Patel J, Levin A, Patel BC. Epiphora clinical testing. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK557424. Last updated July 25, 2023. Accessed July 15, 2024. 61. Dibajnia P, Mohammadinia M, Moghadasin M, et al. Tear film break-up time in bipolar disorder. Iran J Psychiatry. 2012;7(4):191-3. 62. Begley C, Caffery B, Chalmers R, et al. Review and analysis of grading scales for ocular surface staining. Ocul Surf. 2019;17(2):208-20. 63. Eom Y, Lee JS, Keun Lee H, et al. Comparison of conjunctival staining between lissamine green and yellow filtered fluorescein sodium. Can J Ophthalmol. 2015;50(4):273-7. 64. Kim J, Foulks GN. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea. 1999;18(3):328-32. 65. Feenstra RPG, Tseng SCG. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99(4):605-17. 66. Srinivas SP, Rao SK. Ocular surface staining: Current concepts and techniques. Indian J Ophthalmol. 2023;71(4):1080-9. 67. Xiong F, Pula D, Akpek EK, et al. Sjögren’s vs. non-Sjögren’s ocular features: similar symptoms, but significantly worse signs. Invest Ophthalmol Vis Sci. 2024;65(1):23. 68. Hughes WL. Conjunctivochalasis. Am J Ophthalmol. 1942;25(1):48-51. 69. Balci O. Clinical characteristics of patients with conjunctivochalasis. Clin Ophthalmol. 2014;8:1655-60. |
