During the last decade, 14 companies have unsuccessfully attempted to secure FDA approval for a dry eye drug. There are just two underlying explanations for this seemingly insurmountable hurdle: drug ineffectiveness or a flawed approval process.
It is interesting to note that many dry eye agents that failed FDA testing subsequently received approval in Asia and/or Europe, and have achieved tremendous commercial success. For example, since its debut in 1995, topical sodium hyaluronate (Hyalein, Santen) has been the most frequently prescribed dry eye agent in Japan.1
So, what can we learn from the last 10 years of unsuccessful clinical trials? To address the question accurately, we must possess a
better understanding of the FDA approval system, including protocol development, the inclusion/exclusion of signs and symptoms, patient selection and, of course, dry eye disease itself. Only then will we know if future products have a reasonable chance of approval.
Here, we’ll review the inherent difficulties associated with conducting successful FDA testing on potential dry eye drugs. Some of these include appropriate patient selection, the potential for masquerading conditions that can complicate the trial screening process, and the presence of underlying systemic disease that can further exacerbate dry eye signs and symptoms.
Patient Selection
The patient selection process for any FDA trial can be either fairly straightforward or extremely tedious. Certain investigators may have specific patient preferences in mind, depending upon their definition and understanding of the condition being treated. In general, however, the less disease variability that exists between patients, the more rapid and streamlined the selection process.
For example, post-cataract surgery inflammation is one of the most commonly uniform presentations in eye care. Because there is little variability between individual cases, it is relatively simple for researchers to select a population of postoperative cataract patients and evaluate the effectiveness of an anti-inflammatory agent vs. a placebo. Further, because of presentation uniformity, the agent’s effects likely will be consistent as well.
Clearly, however, this is not the case with regard to dry eye disease. When investigators select dry eye patients for FDA trials, they must consider disease type; severity level; and varying contributory factors, such as age, gender, lifestyle and concomitant systemic disease. And, although pharmaceutical companies have well-defined inclusion and exclusion criteria, a wide range of patients often still qualify for the study. Frequently, these are individuals who have failed on other common therapies for dry eye, such as Restasis (cyclosporine, Allergan) or even topical corticosteroids.
So, what’s the result? These patients ultimately are more difficult to treat during the clinical trial, because they have an established history of poor therapeutic response and/or exhibit an underlying systemic condition that requires treatment beforehand.
Types of Dry Eye
As alluded to previously, FDA investigators must consider that there are multiple types of dry eye disease, including evaporative and aqueous deficient. Each form not only exhibits variable severity, but also responds differently to intervention.2 This means that trial researchers must successfully treat patients with widely differing disease patterns.
For example, a long-standing aqueous deficient dry eye patient with Sjögren’s syndrome typically will present with filamentary keratitis and other advanced ocular surface disease findings, such as confluent corneal staining and even scarring of the lacrimal gland.
By contrast, a patient with evaporative dry eye and pronounced meibomian gland dysfunction (MGD) often will not manifest filamentary keratitis or a corneal presentation with severe central staining. Instead, he or she will exhibit obstructed meibomian glands, significant telangiectasia, eyelid inflammation, thickened secretions and notching of the lid margin. Further, this patient may or may not have rosacea.
You cannot realistically expect that both patients, who have markedly different presentations of dry eye disease, will respond similarly to one medication––even though there may be some overlap of clinical findings. And yet, there is a reasonable probability that both individuals would be enrolled in the same clinical trial.
Masquerading Conditions
Perhaps one of the most overlooked reasons for dry eye drug failure in FDA trials is that the patient may not, in fact, have pure dry eye disease. There are many masquerading conditions that either mimic dry eye or can present concomitantly. Either way, masquerading conditions may complicate the results or prohibit complete resolution of the signs and symptoms––even if the dry eye component is treated successfully with the tested agent.
• Conjunctivochalasis. Patients with conjunctivochalasis often report foreign body sensation, grittiness, irritation and tearing, and may be diagnosed with dry eye disease. Such patients typically will have signs of dry eye and inflammation, and may even show a rapid tear film break-up time, corneal staining and lissamine green conjunctival staining. But, clinical trial recruiters often mistake these signs and symptoms for true dry eye. And unfortunately, no treatment––other than a surgical repair of the conjunctiva––will yield significant sign or symptom improvement.

Recurrent corneal erosion, as seen in this patient, is one of the most common dry eye masqueraders observed in clinical practice.
Most patients with conjunctivochalasis actually can pinpoint where the foreign body or pain emanates from, which typically matches the location of the conjunctival folds. If the patient is able to pinpoint the foreign body sensation that matches the area of conjunctivochalasis, he or she should not be included in a dry eye trial.
Patients with long-standing inflammatory conditions, such as dry eye and allergic conjunctivitis, are prone to develop conjunctivochalasis. Additionally, research has shown a possible association between conjunctivochalasis and immune thyroid disease. A perspective study published in 2006 found that the prevalence of conjunctivochalasis in patients on immune thyroid disease was as high as 88%.3
• RCE. Another condition that can masquerade as dry eye disease is recurrent corneal erosion (RCE). In these patients, poor adhesion of the hemidesmosomes between the epithelial basement membrane and Bowman’s layer results in symptoms of grittiness, dryness, photophobia, tearing, foreign body sensation, blurred vision and, in some instances, irritation or pain.
• EBMD. Individuals with epithelial basement membrane dystrophy (EBMD), but no RCE, can display symptoms that mimic dry eye. However, EBMD is not likely to improve with the use of many therapeutic agents. Even with treatment, the presence of maps, dots and fingerprints and staining patterns will remain in patients with anterior corneal dystrophies.
Further, I have reviewed few FDA testing protocols for dry eye that specifically differentiated between symptoms documented at the beginning of the day vs. those observed at the end of the day. From clinical experience, it seems that the symptoms of MGD, RCE and EBMD occur with greater frequency in the morning, while symptoms associated with pure dry eye typically manifest as the day progresses. For example, seeing a patient in the morning for a study visit may elicit a different complaint than a late-day exam.
• MFS. Another condition that may present similarly to dry eye disease is mucin fishing syndrome (MFS). Like dry eye, MFS is a chronic condition that causes patients to extract or “fish” strands of mucin from their eyes.
The condition may begin with potential dry eye disease resulting in significant mucin production. The excess mucin causes a visual disturbance and associated symptomatology that is similar to that documented in dry eye.
When patients repeatedly touch their eyes to remove the mucin, they further irritate the ocular surface as well as potentially introduce foreign substances to the eye.4 Eventually, patients with MFS begin to dig in their eyes habitually. And, as long as fingers continue to come in contact with the eyes, the individual’s symptoms will persist––regardless of treatment.
Once again, MFS typically is not considered in the exclusion criteria for any dry eye trial. What makes this even more difficult is that dry eye likely is one of the most common underlying etiologies of MFS, and therefore affected patients likely will be enrolled in a clinical trial for a dry eye medication.
• FES. Athough it is a relatively uncommon condition, floppy eyelid syndrome (FES) rarely is included in the exclusion criteria of any dry eye trial. This is because few investigators evert patients’ eyelids prior to study enrollment. FES patients often manifest significant ocular surface complications, including advanced superficial punctate keratopathy (SPK), rapid tear film break-up time and conjunctival staining. Further, FES patients typically present with chronic SPK; advanced MGD; and symptoms of burning, stinging, irritation and chronic conjunctival injection.
FES is most common in overweight, middle-aged males who suffer from sleep apnea.5 Because such individuals have limited eyelid functionality, they have chronic ocular surface inflammation. Furthermore, patients with FES often report spontaneous lid eversion.6 So, for the purposes of a dry eye investigation, a simple questionnaire might capture this potential finding prior to study enrollment.
Once again, any tested medication is not likely to improve the patient’s condition––even though he or she may have many signs and symptoms of dry eye. Unless the upper eyelid is surgically repaired, the symptoms likely will persist.
• GPC. Giant papillary conjunctivitis (GPC) is another dry eye masquerader that presents with symptoms of grittiness, irritation, mucin discharge and decreased contact lens wear. Similar to the diagnosis of FES, upper eyelid eversion during the screening examination process will help reveal this condition as well.
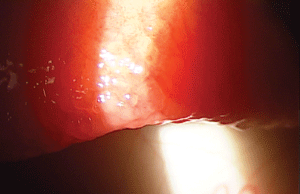
It is imperative to evert the upper eyelids when screening dry eye patients for giant papillary conjunctivitis, as seen here.
• Salzmann’s. Patients with Salzmann’s nodular degeneration (SND) often are enrolled in clinical dry eye studies. In certain individuals, SND’s presentation can be quite subtle and may appear as a whitish peripheral haze––not frank nodules. What makes this confusing is that patients with SND exhibit dry, gritty, irritated eyes as well as an associated foreign body sensation, transient blur and rapid tear film break-up time.7
Approximately 75% to 90% of all SND cases manifest in white females.8 This complicates any trial screening process, because dry eye disease is most prevalent in this demographic. Additionally, nearly 63% of all SND cases occur bilaterally––further resembling a presentation of dry eye.9
Confocal microscopy reveals that SND lesions are elongated basal epithelial cells and activated keratocytes, particularly in the area of the anterior stroma near the nodules.10 Occasionally, sub-basal nerves and tortuous stromal nerve bundles may be observed. Ultrahigh-resolution optical coherence tomography has uncovered fibrous intraepithelial nodules with significant overlying epithelial thinning in SND patients.11 This finding may contribute to symptoms that mimic those associated with dry eye disease; however, they will not respond to agents designed to treat ocular surface disease.11
• Asthenopia. One of the most important and frequently disregarded dry eye masqueraders is asthenopia, described as a collection of conditions that induce ocular fatigue. Affected patients rarely are screened for any clinical trial, and yet demonstrate several symptoms that overlap with dry eye disease. Many patients also may have concurrent dry eye––but if their symptoms are caused by asthenopia, they will not respond fully to the study medication.
Common asthenopic conditions include computer vision syndrome, convergence insufficiency, proprioceptive disparity, fixation disparity, and even exophoria and vertical disparities. Patients with asthenopia typically complain of ocular ache or pain; dryness, redness, grittiness and burning; excessive tearing; visual fatigue with near work, such as computer use; and headaches or an uncomfortable “pulling sensation.”12 The sweeping majority of these symptoms are identical to those experienced by an individual with dry eye.
Patients with dysphoria, fixation disparity, proprioceptive disparity and/or a vertical imbalance between the two eyes often will complain of dryness, grittiness, ocular irritation, visual fatigue, blurred vision and headaches. Many patients who have one of these conditions will test negative for dry eye and will be excluded from a clinical trial. However, in some instances, these individuals will have concomitant dry eye symptoms and will be enrolled in the trial. All too often, however, these symptoms will not resolve with use of the study medication, because the underlying condition is unrelated to dry eye disease.
Limited Correlation Between Signs and Symptoms
Over time, dry eye patients typically experience corneal nerve fiber damage and a subsequent loss of sensitivity. Thus, as a patient’s condition progresses, he or she actually may exhibit fewer dry eye symptoms. This association can make proper patient selection very challenging.
Research conducted by Kelly K. Nichols, OD, MPH, PhD, and associates suggested that the signs and symptoms of advanced dry eye disease do not always correlate.13 Another study indicated that 40% of dry eye patients do not manifest symptoms.14 Furthermore, patients with neurotrophic dry eye typically will have advanced signs, but few if any symptoms other than blurred vision.14
Relying upon the presence of corneal staining as an indicating sign of dry eye disease also may be problematic for trial researchers. If corneal staining is selected as a screening parameter, many early dry eye patients will be excluded. When corneal staining becomes clinically evident, dry eye disease is fairly advanced. This is similar to the presence of visual field defects during a glaucoma screening––by the time a clinician notes a defect, the nerve fiber layer already has been damaged.
Underlying Disease and Systemic Drug Use
Many dry eye patients who are potential candidates for a clinical trial may have contributory underlying systemic diseases. Further, the medications used to control these conditions can further exacerbate ocular dryness. And, as long as patients use these medications
and/or have poor control of the underling systemic condition, they will continue to exhibit dry eye symptoms––often irrespective of topical intervention.
Some patients with long-standing acne rosacea, for example, also have advanced MGD and associated dry eye disease. In these instances, patients will pass many of the entrance tests required for a dry eye trial. However, because the meibomian glands are so scarred and damaged, very few––if any–– topical agents will modify this condition. Instead, the patient will require a systemic agent, such as doxycycline and/or mechanical treatments to control the underlying rosacea and achieve an optimal result.
Additionally, we often see chronic staining and even persistent epithelial defects in patients diagnosed with type 2 diabetes
mellitus. Until those individuals improved glucose control with insulin therapy, very few topical agents effectively treated their dry eye.
Furthermore, patients who are on multiple systemic medications, including diuretics or antihistamines, are much more likely to have dry eye symptoms. As long as these systemic medications are being used, patients in a dry eye trial will experience a limited response to the drug being tested. Patients who are on more than three systemic medications should be excluded from dry eye clinical trials.15
Undocumented Disease Variability Between the Eyes
I have been working in the field of dry eye management for my entire career, which now spans close to 20 years. Even after two decades, I’ve realized that we still have a lot more to learn about the overarching complexity and varying presentation of dry eye disease.
For instance, just within the last few years, we’ve begun to notice that dry eye patients often demonstrate bilateral symptom variability. In other words, it appears that patients with dry eye often experience compensating effects between the two eyes, such as more pronounced staining or increased tear film osmolarity in one eye vs. the other.
This makes accurate clinical testing of both eyes critical. For example, it is imperative for trial researchers to examine both eyes independently (e.g., not to average Schirmer readings, osmolarity scores or tear film break-up times together, but instead document the highest measurements for each eye individually). Bottom line––a more comprehensively thorough method to measure dry eye severity in FDA trials likely will be required for new dry eye drugs to demonstrate marked success and receive approval in the future.
During the next decade, some dry eye medications undoubtedly will receive FDA approval––despite many of the difficulties and limitations outlined above. However, the medications likely will have to be exceptional. Because dry eye disease is one of the most common ocular conditions in the United States, it should not be extremely difficult to populate new, more tightly regulated clinical trials. Because such trials could be both costly and lengthy, it would be ideal to focus upon the key variables outlined in this article––without complicating trial recruitment too extensively.
These issues notwithstanding, new and more effective therapeutic agents for dry eye disease are needed desperately. Hopefully, these recommendations will facilitate success in future FDA trials and, most importantly, provide your dry eye patients with greater relief.
Dr. Karpecki is the clinical research director at Koffler Vision Group in Lexington, Ky. He is a paid consultant to SARcode Biosciences, but has no direct financial interest in any products mentioned.
1. Nishihata T. Santen Pharmaceutical Co., Ltd. Fulfilling unmet opthalmic treatment needs: Contributing to dry eye treatment. Available at:
www.santen.com/ir/reports/ar2011_03.pdf. Accessed December 19, 2012.
2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
3. de Almeida SF. Clinic-cytologic study of conjunctivochalasis and its relation to thyroid autoimmune diseases: prospective cohort study. Cornea. 2006 Aug;25(7):789-93.
4. Slagle WS. Mucus fishing syndrome: case report and new treatment option. Optometry. 2001 Oct;72(10):634-40.
5. Pham TT, Perry JD. Floppy eyelid syndrome. Curr Opin Ophthalmol. 2007 Sep;18(5):430-3.
6. Karger RA, White WA, Park WC, et al. Prevalence of floppy eyelid syndrome in obstructive sleep apnea-hypopnea syndrome. Ophthalmology. 2006 Sep;113(9):1669-74.
7. Singer AR, Pahl S. A familial anterior corneal degeneration: clinical aspects, histopathology and differential diagnosis. Klin Monbl Augenheilkd. 1998 Aug;213(2):104-7.
8. Hamada S, Darrad K, McDonnell PJ. Salzmann’s nodular corneal degeneration (SNCD): Clinical findings, risk factors, prognosis and the role of previous contact lens wear. Cont Lens Anterior Eye. 2011 Aug;34(4):173-8.
9. Fario AA, Halperin GI, Sved N, et al. Salzmann’s nodular corneal degeneration clinical characteristics and surgical outcomes. Cornea. 2006 Jan;25(1):11-5.
10. Roszkowska AM, Aragona P, Spinella R, et al. Mor- phologic and confocal investigation on salzmann nodular degeneration of the cornea. Invest Ophthalmol Vis Sci. 2011 Jul 29;52(8):5910-9.
11. Hurmeric V, Yoo SH, Karp CL, et al. In vivo morpho- logic characteristics of Salzmann nodular degeneration with ultra-high-resolution optical coherence tomography. Am J Ophthalmol. 2011 Feb;151(2):248-56.e2.
12. Mayo Clinic staff. Eyestrain Causes. Mayo Clinic. 2010 Jul. Available at:
www.mayoclinic.com/health/eyestrain/ DS01084/DSECTION=causes. Accessed August 2012.
13. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004 Nov;23(8):762-70.
4. Lemp MA. Clinical trials in dry eye in surgery for dry eye? Dev Ophthalmol. 2008;41:283-97. doi: 10.1159/000131096.
15. Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851.
It has been more than a decade since the
first and only dry eye prescription medication, Restasis, received FDA
approval. And while pharmaceutical companies have had tremendously
limited success bringing a new drug to the US market, excitement is now
brewing as we anticipate the approval of several new therapeutic agents
for dry eye disease within the next few years.
• Cyclokat (0.1% cyclosporine A cationic emulsion, Novagali Pharma)
• Lifitegrast.
SARcode Bioscience completed Phase III testing of this first-in-class,
highly selective, small-molecule, integrin antagonist in October 2012.
Lifitegrast inhibits the binding of two key surface proteins—lymphocyte
function-associated antigen-1 and intercellular adhesion molecule
(LFA-1/ICAM-1)—that are integral to chronic T-cell mediated
inflammation. This action prevents T-cell migration, adhesion,
proliferation and cytokine release, which are all implicated in dry eye
disease. In the Phase III
study, lifitegrast 5.0% ophthalmic solution demonstrated a superior
reduction in the signs and symptoms of dry eye disease vs. placebo.1
It significantly improved inferior and total corneal staining scores
from baseline, and markedly reduced both ocular discomfort and dryness.
Following
completion of the Phase III trial, SARcode Bioscience initiated a
year-long safety study (SONATA), and will soon begin a second Phase III
confirmatory study (OPUS-2).2 Data from both SONATA and OPUS-2 will be used to support the filing of a New Drug Application. • CF101.
OpthaliX, the US subsidiary of Israel’s Can-Fite BioPharma, is
currently evaluating this first-in-class A3 adenosine receptor agonist
in a Phase III trial. A3 adenosine receptors are involved in a variety
of intracellular signaling pathways and physiological functions. These
receptors also help to inhibit degranulation in neutrophil-mediated
tissue injury. Further, CF101 modulates key signaling proteins that
inhibit inflammatory cytokine/chemokine production and induce
inflammatory cell apoptosis.
CF101
is administered orally, and has been tested for the treatment of dry
eye disease, glaucoma and uveitis in Phase II studies.3 The
drug also is being evaluated for the treatment of autoimmune
inflammatory diseases, including rheumatoid arthritis (Phase IIb) and
psoriasis (Phase II/III). • Rebamipide. This
gastroprotective drug stimulates endogenous prostaglandin generation in
the gastric mucosa, scavenges free radicals, and is reported to
accelerate ulcer healing.4 In 1990, Japan’s Otsuka
Pharmaceutical Company first marketed rebamipide as Mucosta tablets for
treating gastric lesions and ulcers.
In January 2012, Otsuka launched Mucosta ophthalmic suspension (2.0% rebamipide) as a novel dry eye treatment in Japan.5 The drug acts to increase the level of mucin in the tear film.
In
July 2012, Otsuka and Acucela announced the initiation of a Phase III,
multi-center, randomized, placebo-controlled, double-masked,
parallel-group study clinical trial to evaluate Mucosta ophthalmic
suspension in patients with dry eye syndrome.5 The companies anticipate the trial to be completed by the end of 2013.
• MIM-D3.
In November 2012, Canada’s Mimetogen Pharmaceuticals Inc. announced
that it received a US-issued patent for MIM-D3—a first-in-class,
small-molecule nerve growth factor receptor (TrkA) agonist used to treat
dry eye disease. MIM-D3 is a mimetic of nerve growth factor (NGF) that
binds specifically to the TrkA receptor.
NGF
is a naturally occurring protein found in the eyes that is responsible
for the maintenance of the corneal nerves and epithelium, mucin and tear
production. NGF shows a potential benefit in dry eye disease
management, including neurotrophic effects, corneal healing and mucin
secretion.
In June 2011,
Mimetogen completed a Phase II, randomized, double-masked, multi-center,
placebo-controlled trial designed to evaluate the safety, tolerability
and efficacy of MIM-D3. The trial results showed that patients exhibited
improved signs and symptoms of dry eye disease as well as excellent
safety and tolerability profiles.6
• RGN-259.
RegeneRx Biopharmaceuticals is developing RGN-259––a thymosin beta
4-based, preservative-free eye drop––as a novel treatment for corneal
healing in patients with moderate to severe dry eye disease. Thymosin
beta 4 is a naturally occurring peptide found in high concentrations in
blood platelets, wound fluid and other tissues. In
June 2012, results from a Phase II trial showed that RGN-259
significantly improved several signs and symptoms of dry eye in 72
patients.7 In separate studies, RGN-259 effectively promoted
corneal healing in patients with chronic, medically unresponsive,
non-healing corneal defects secondary to loss of corneal innervation
(primarily associated with diabetes and herpes zoster).8
• Rivoglitazone.
Santen Pharmaceutical’s rivoglitazone is a peroxisome
proliferator-activated receptor gamma agonist contained in an ophthalmic
solution that currently is under investigation for the treatment of dry
eye. Peroxisome proliferator-activated receptors represent a group of
nuclear receptor proteins that function as transcription factors that
regulate the expression of genes.
Santen
has initiated Phase II trials of rivoglitazone for the treatment of
corneal and conjunctival epithelial disorders associated with dry eye.9
The company’s researchers believe that rivoglitazone enhances the
barrier function of the corneal epithelium.9 • RX-10045.
This synthetic resolvin analog, developed by Resolvyx Pharmaceuticals,
is formulated for topical application to treat dry eye disease. In early
studies, RX-10045 has promoted tissue repair of human corneal
epithelial cells in vitro.10
Resolvins,
a group of lipid modulators derived from omega-3 fatty acids, are
naturally occurring, small molecules that protect healthy tissue during
an immuno-inflammatory response. Additionally, these lipid modulators
help to resolve inflammation and promote healing, permitting inflamed
tissues to return to homeostasis once the insult has passed. Currently,
Rx-10045 is in Phase II testing.10 • EBI-005. At
the 2012 ARVO Annual Meeting in Fort Lauderdale, Fla., researchers from
Eleven Biotherapeutics presented preclininical data on EBI-005, a
topically-applied interleukin-1 (IL-1) receptor antagonist protein.11
Targeting IL-1 is a promising therapeutic approach for the treatment
dry eye, because it is a critical mediator of the inflammatory cascade
associated with the symptoms of ocular surface disease. In
early December 2012, the company announced initiation of a Phase 1b
clinical trial of EBI-005, and it expects to conclude this study during
the first half of 2013.12 Dr. Mastrota is the center director at Omni Eye Surgery in New York.
A Glimpse at Dry Eye Drugs in the Pipeline
By Katherine M. Mastrota, MS, OD
Currently,
there more than a dozen novel, innovative drugs in FDA trials. Any one
of these medications could be the next big breakthrough product used to
treat your dry eye patients. Here is a review of the most promising dry
eye medications across various phases of development:
Other Potential Dry Eye Drugs in the Pipeline
• Restasis X (0.1% cyclosporine A, Allergan)
• CP-690560 (tofacitinib, Pfizer)
• LX214 (voclosporin, Isotechnika Inc./Lux Biosciences Inc.)
• ISV-101 (bromfenac in DuraSite vehicle, InSite Vision)
2. Semba C. Safety study of lifitegrast to treat dry eye (SONATA). Clinical Trials Identifier: NCT01636206 Available at:
http://clinicaltrials.gov/ct2/show/NCT01636206. Accessed January 3, 2013.
3.
Avni I, Garzozi HJ, Barequet IS, et al. Treatment of dry eye syndrome
with orally administered CF101: data from a phase 2 clinical trial.
Ophthalmology. 2010 Jul;117(7):1287-93.
4. Arakawa T, Kobayashi K,
Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of
action and efficacy in mucosal protection and ulcer healing. Dig Dis
Sci. 1998 Sep;43(9 Suppl):5S-13S.
5. Otsuka Pharmaceutical Company,
Ltd. Acucela and Otsuka Pharmaceutical announce the initiation of a
Phase 3 clinical trial to evaluate rebamipide ophthalmic suspension in
patients with dry eye syndrome. Available at:
www.otsuka.co.jp/en/release/2012/0719_02.html. Accessed January 3, 2013.
6. Medwell Capital Corp. Mimetogen Phase 2 data for MIM-D3 presented at leading ophthalmology conference. Available at:
www.medwellcapital.com/display-press-release.php?id=240. Accessed January 3, 2013.
7.
RegeneRx Biopharmaceuticals, Inc. RGN-259 significantly improves signs
and symptoms of severe dry eye in Phase 2 clinical trial.
www.regenerx.com/wt/page/pr_1340196791. Accessed January 3, 2013.
8.
Dunn SP, Heidemann DG, Chow CY, et al. Treatment of chronic nonhealing
neurotrophic corneal epithelial defects with thymosin beta 4. Arch
Ophthalmol. 2010 May;128(5):636-8.
9. Santen, Inc. Study assessing
safety and efficacy of DE-101 ophthalmic suspension in dry eye patients.
Clinical Trials Identifier: NCT01118754. Available at:
www.clinicaltrials.gov/ct2/show/NCT01118754. Accessed 12/24/2012. Accessed January 3, 2013.
10.
Torkildsen G. A study of RX-10045 in the treatment of dry eye disease.
Clinical Trials Identifier: NCT01675570. Available at:
http://clinicaltrials.gov/ct2/show/NCT01675570. Accessed January 3, 2013.
11.
Eleven Biotherapeutics. Eleven Biotherapeutics presents data on
EBI-005, a novel IL-1 inhibitor protein for topical treatment of dry eye
disease. Available at:
www.elevenbio.com/pdfs/releases/2012%20ElevenEBI-005Data%200508. Accessed January 3, 2013.
12.
Eleven Biotherapeutics. Eleven Biotherapeutics initiates Phase 1b
clinical study of EBI-005, a novel, topically-delivered ILB1 inhibitor
protein for the treatment of dry eye disease. Available at:
www.elevenbio.com/pdfs/releases/2012%20Eleven%20EBI005%20Ph1bStart%20121012.pdf. Accessed January 3, 2013.

