Visual Fields in the Era of OCT
Is functional testing with visual fields still necessary in the age of advanced structural imaging with optical coherence tomography?
By Lauren Ristin, OD, and Andrew Rixon, OD
Release Date: May 15, 2019
Expiration Date: May 15, 2022
Estimated Time to Complete Activity: 2 hours
 |
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Explain why OCT is generally more sensitive than VF in detecting progression in early glaucoma, but not in moderate and advanced glaucoma.
- Interpret measurement of the peripapillary retinal nerve fiber layer, particularly in early glaucoma.
- Determine thickness of the macular ganglion cell complex to monitor progression from early to advanced glaucoma.
- Evaluate differences or inconsistencies between VF and OCT results.
- Incorporate VF and OCT as complementary tests for diagnosing, following and managing patients with glaucoma.
Target Audience: This activity is intended for optometrists engaged in the care of patients with glaucoma.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Lauren Ristin, OD, Jesse Brown Veterans Affairs Medical Center (VAMC) in Chicago, IL, and Andrew Rixon, OD, Memphis VAMC.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 62380-GL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Drs. Ristin and Rixon have nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
Early and optimal detection of glaucoma and its progression is critical in preventing a burden on optometry’s patients and to society as a whole. The difficulty arises in how to optimally detect and gauge glaucomatous progression. Traditionally, we were limited to functional testing of visual fields (VF) using standard automated perimetry (SAP). Although this is well understood and widely employed technology, the evolution of optical coherence tomography (OCT) gives us the ability to consistently gauge structural change. The major, unresolved questions now are whether OCT technology should supplant VFs in detecting progression, whether visual field testing still takes precedence, whether the two are mutually exclusive, or whether integrating them is optimal for glaucoma care.
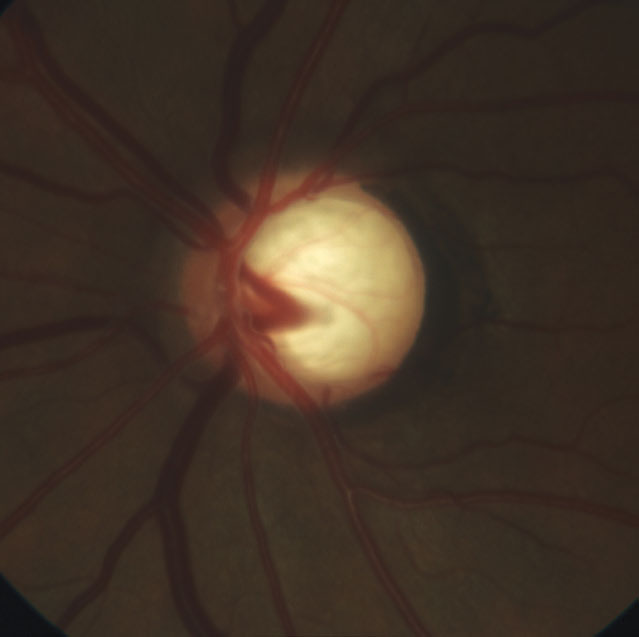 |
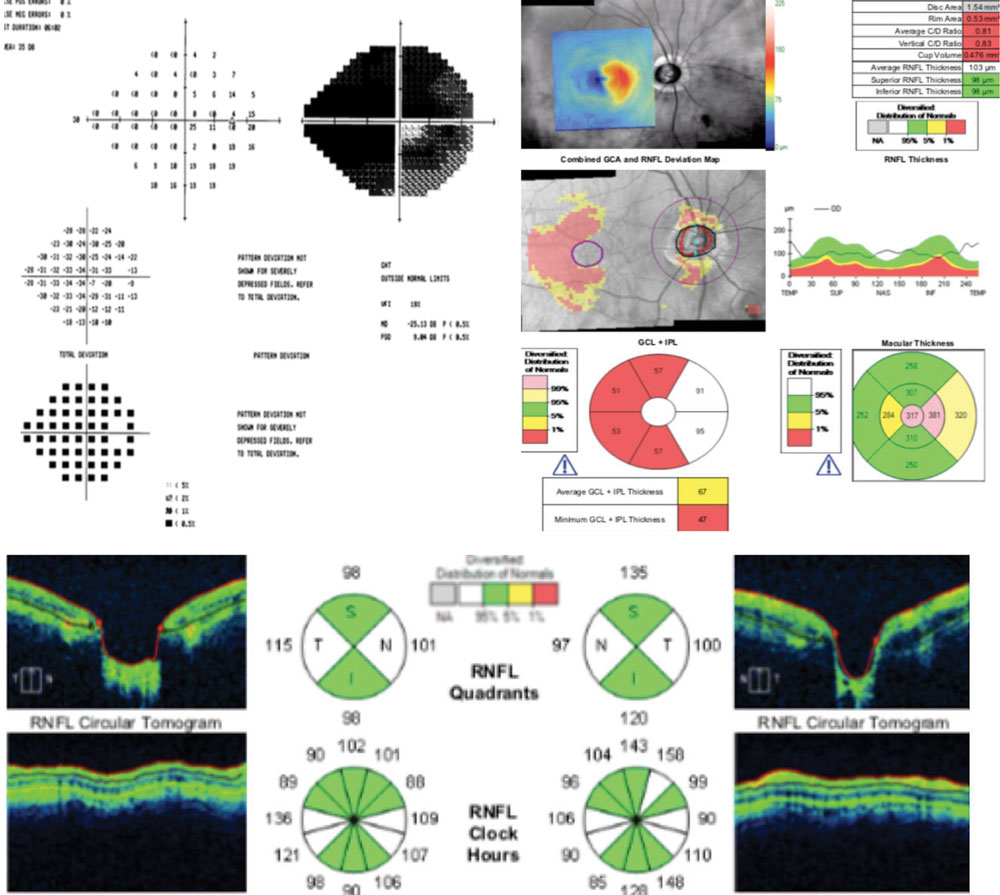
| This 51-year-old patient’s optic nerve shows extensive vertical neuroretinal rim loss and deep laminar cupping with laminar reconfiguration. |
Structure-Function Relationship
Ultimately, both structural and functional tests, and the progressive changes gauged by those tests, are related to the pathological loss of retinal ganglion cells (RGCs) and their axons. The method in which current VF and OCT technologies measure the RGCs and their loss differs, leading to the often discordant structure-function (S-F) relationship, which is tied to various assumptions about which of the two technologies is superior for glaucoma management.
Speaking of technology, although research shows VF testing alternatives such as frequency doubling technology and flicker defined form perimetry have stronger correlation between S-F when compared with SAP, SAP is still the most frequently used form of perimetry and will be the form discussed in this structure-function conversation.1-3
Significant structural change in early glaucoma—traditionally defined by SAP perimetry and, for the purposes of this article, specifically referring to Hodapp-Parrish-Anderson criteria (HPA)—is equivalent to much smaller relative functional change.4 In advanced disease, the same amount of structural change results in substantial functional change.5 As such, the S-F relationship is generally curvilinear, with retinal nerve fiber layer (RNFL) thickness becoming linearly related to SAP in moderate to advanced (per HPA) disease states.6
One estimate of this curvilinear relationship indicated that a loss of 100,000 RGCs in early glaucoma (baseline average mean deviation [MD] of -2 decibels [dB]) would result in a 1.79dB MD change on VFs. However, that same loss of 100,000 RGCs in a severe stage case (average MD of -15dB) would result in a 5.78dB MD change.7
As a result, OCT is often favored over VFs in early disease, and multiple studies do support its superiority over SAP at that stage.8 OCT has been shown to detect glaucomatous change on RNFL scans up to eight years prior to detection by VFs—in 19% of patients in one study.9
However, once the patient’s disease reaches a moderate or severe level, functional testing may surpass OCT as the best method to detect progression because OCT may under-sample damage at that stage. Researchers explored OCT’s ability to sample tissue thickness throughout the spectrum of disease, and estimated that a loss of 100,000 RGCs in early glaucoma (baseline average MD of -2dB) would result in a 5µm change on OCT. That same 100,000 RGC loss would result in just a 1.5µm change on OCT at a severe level.7
Ultimately, OCT testing is limited by the “floor effect”—when average RNFL measurements are approximately 50µm (a number that varies by platform).10 At this level, RNFL tissue is no longer discernible. Although the machine will capture some quantity of tissue, it is thought to be non-neural in nature.11 This has led to the notion that OCT is substandard in severe stages of glaucoma and visual fields alone are useful in monitoring progression
in advanced states.
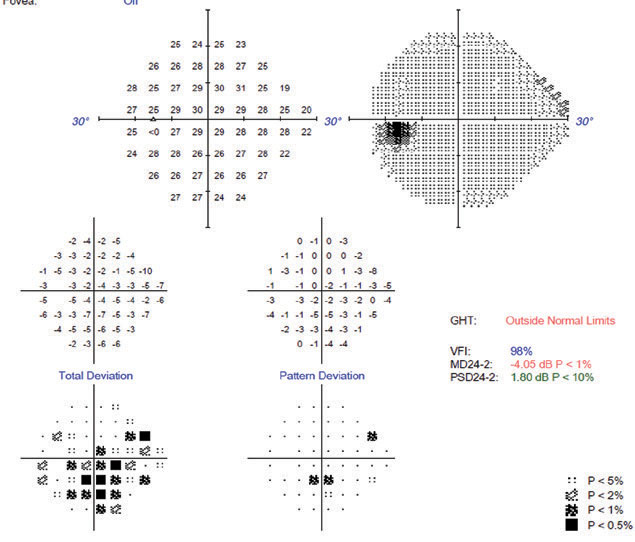 |
| The patient’s 24-2 VF is not as dense as expected based on the damage found on clinical and OCT evaluation. The pattern deviation’s correction of diffuse changes masks the widespread field defect shown by the total deviation plot. |
But this is not entirely accurate.
We know that the macula is involved at all stages of glaucoma while the papillomacular bundle is more resistant to damage in advanced disease when compared with RNFL.12 Indeed, measuring the ganglion cell-inner plexiform layer (GC-IPL) shows the potential to identify structural progression not only in the early stage but also in late glaucoma, potentially extending the use of OCT into severe cases.
Why then does RNFL damage often “precede” functional damage seen on 24-2 VFs?
The answer is rooted in the difference between the two technologies. OCT (depending on the platform used) quantifies neuroretinal rim thickness (NRR), ganglion cell layer (GCL), RNFL, GC-IPL and total macular thickness (TMT) in linear units (mm2 for NRR and µm for RNFL and respective macular thickness measurements). Subsequently, when RGCs die, GC-IPL, GCL, TMT, RNFL and NRR should also decrease linearly. A caveat to this is that RGC thicknesses vary depending on proximity to the foveal center and recent research shows isolation of both GCL and IPL can have diagnostic value.13-16 The ability to capture the RGC bodies (GCL) and their dendrites (IPL) will vary across platforms.
Visual field sensitivity, however, is recorded in decibels, which are logarithmic units. Decibels do not express an absolute quantity, as linear µms do, but rather a ratio based on the maximum amount of background luminance provided by the specific perimetry unit employed. As an example of this logarithmic nature, a change of 3dB may actually represent a halving or doubling of light intensity.17
To complicate matters, values across different perimeters are not comparable due to differing maximal output stimuli and background luminance, meaning that a certain dB value on one perimeter is not equal to the same dB value on another.18 Clearly, misalignment of the structural and functional measurement units results in discord. Interestingly, studies show that when perimetric sensitivity is expressed in linear units, there is good alignment with RGC density in the retinal region tested.17 This underscores that the actual, real-world S-F relationship is complex and should not be oversimplified.
The detection ability of each of these technologies will vary according to the individual patient, and presumptions about structure preceding function do not apply to every patient.19
Specific VF-related factors that influence the discord and presumption that OCT is better in early disease, and VFs are better in more severe stages, include:
- The redundancy of the visual system, which results from the ability of RGCs neighboring dead or damaged RGCs to compensate for those cells. Any SAP stimulus projected onto a particular retinal region affects many different RGCs. Their redundancy is thought to prevent detection of functional change until most of the RGCs are no longer functional, rendering SAP insensitive in early disease.6
- Substantial intra- and inter-subject variability in SAP testing.
- Inadequate assessment of retinal loci damaged by glaucoma with standard 24-2 testing.
- The concept that RGC dysfunction precedes RGC death.6,20
Visual field variability is the result of the subjective, psychophysical nature of the test and is well known to have intra-subject test-retest variability, which only increases intra- and inter-subject variability, confounding diagnosis and assessment of disease progression with SAP.17 Conversely, it is assumed that OCT, given its objective nature, exhibits low measurement variability and should therefore be more precise in determining change.
But it’s not entirely clear whether this assumption holds true for all patients.21 Recently, researchers confirmed the above assumption, determining that eyes with less severe disease (less than -10dB average MD) had a higher likelihood of having worsening disease detected by spectral-domain OCT than SAP, and those with more severe disease had a higher chance of being detected by SAP. However, they also found that progression could be detected by either method in all states of glaucoma, reinforcing that although OCT may be more precise, on average, than 24-2 SAP in early disease, this may not be the reality for the specific patient sitting across from you.10
Another S-F presumption is that measuring tissue thickness is a surrogate way to measure RGC dysfunction.17 RGCs exhibit a period of dysfunction prior to their death that structural measurements may not capture. For any given patient with the same RNFL thickness, a functional defect may arise prior to flagged structural loss, depending on RGC dysfunction.6 This issue is highlighted by researchers who sought to determine the average RNFL thickness level where VF loss becomes manifest. Using Cirrus HD-OCT (Zeiss), the group found that the “tipping point” at which functional loss manifested was an average of 75.3µm.22
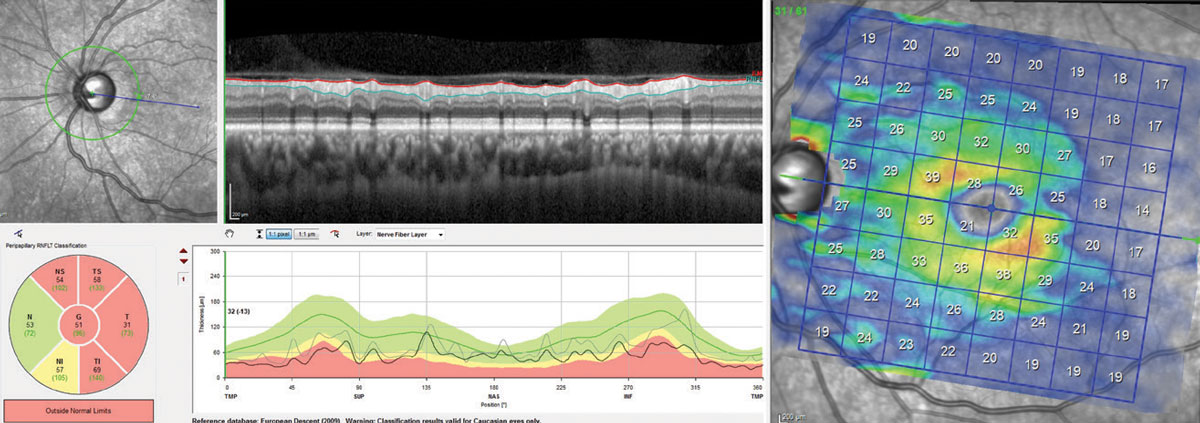 |
| Cp-RNFL, at left, and isolated GCL, at right, shows RNFL tissue at “floor” (average RNFL of 51µm). The segmentation scan shows that the majority of the tissue thickness comes from the blood vessels, not neural tissue. Although a double arcuate loss of tissue exists on the GCL scan, there is sufficient tissue to track structural progression, whereas the RNFL scan is no longer useable. Click image to enlarge. |
More detailed analysis of the data showed equal levels of VF loss at RNFL averages ranging from approximately 90µm to 50µm, signifying that RGC dysfunction might be captured by VF testing with presumably normal RNFL thicknesses. Of course, we have no way of knowing what our patient’s RNFL thicknesses were prior to their initial visit. Baseline normals will vary, and an individual may start with a thicker or thinner RNFL, which will cause their individual “tipping point” to vary.22
A similar study using Spectralis OCT (Heidelberg) found the tipping point was an average of 89µm.23 Analysis of the data showed a similarly wide range of RNFL levels where equal VF loss occurred. This reinforces that different patients and different instruments may show different levels of progression. RGC damage may be captured first by functional testing in spite of our preconceived notions of an abnormal RNFL thickness.
With the discordant S-F relationship sometimes favoring OCT and sometimes favoring VFs, the only consistent expectation we should have is that functional and structural progression will not be detected simultaneously, and if they do, will do so infrequently. So, if you see progression on structural testing during one visit, don’t expect to confirm such progression on functional testing on that same visit, and vice-versa.24,25
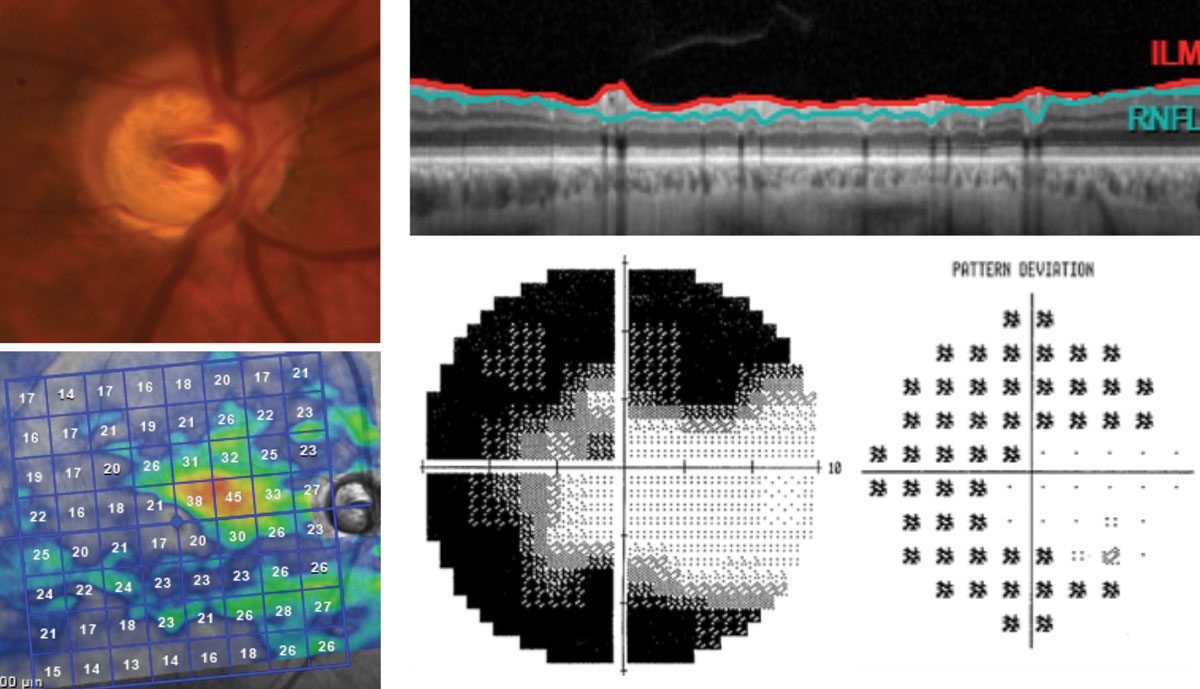 |
| The right eye of this 65-year-old patient has severe stage glaucoma post-trabeculectomy. The segmentation scan, top right, shows no discernible RNFL thickness. The grayscale and pattern deviation plots, bottom right, show some remaining inferotemporal field on 10-2 testing. This corresponds well with the remaining superior nasal tissue found on isolated GCL analysis. Click image to enlarge. |
OCT Pointers and Pitfalls
RNFL thickness and thickness deviation maps, as well as macular thickness parameters, have been shown to objectively discriminate between healthy and glaucomatous eyes, especially early in the disease.26 However, these machines are not without limitations.
10-2 + 24-2 = 24-2C?Because the earliest damage from glaucoma involves local damage to the macula, some glaucoma specialists have advocated for performing a 10-2 visual field test in addition to a 24-2.44 The clinical logistics of this suggestion are not ideal, so Zeiss recently developed the 24-2C test pattern for its HFA3 perimeter. It remains to be seen if this new strategy will improve structure-function agreement in early disease. |
Awareness of the realities and pitfalls of OCT technology is important to avoid misinterpretation. While all major OCT platforms capture circumpapillary RNFL (cpRNFL) using a similar sized measurement circle (3.46mm in diameter) and have been found to have similar abilities to help the clinician detect change, each is unique in its axial properties, axial resolution, segmentation algorithm and image processing capabilities. However, there is no standardization across platforms, which prevents direct comparison of measurements amongst them.27,28
When interpreting the peripapillary RNFL thickness parameters, there are a number of factors that a clinician needs to consider prior to determining whether their patient has glaucoma. First, attrition of RGCs is a normal part of the aging process and is expected. In OCT, the normal age-related attrition of RNFL varies according to the report, with recent studies showing an average global loss ranging from 0.33µm/year to 0.54µm/year.27,29 Each instrument accounts for these natural changes by comparison with an age-matched normative database. While these databases are not exhaustive, a recent study has shown both the Spectralis and Cirrus databases successfully identified healthy patients.
 |
| This patient has advanced glaucoma in the right eye, which is masked on the RNFL and ganglion cell analysis by a large ERM between the ONH and the fovea (visible on the ganglion cell analysis printout), leading to anomalously normal “green” values. Artifacts should always be accounted for in analysis. |
But their specificity is much lower when trying to identify those who will develop glaucoma.30
Normal population-based RNFL thickness variability is likely to blame for the reduced specificity. The database reports “normal” tissue using a green color code. This normal tissue is found within a dynamic range of tissue that represents 90% of patients in the reference database. Patients born with greater amounts of tissue compared with the reference database may have legitimate glaucoma but can be falsely flagged as being normal due to how the machine presents the data.31 As such, substantial loss could occur within the normal range.29
The recognition of OCT scan artifacts and sources of error creating them is critical to accurately interpreting the data. Multiple sources of error exist and can generally be divided into patient-dependent, operator-dependent and device-dependent.
One of the most common causes of patient-dependent artifacts in both the RNFL and ganglion cell complex (GCC) is the presence of an epiretinal membrane.32,33 In one review, the upper boundary of the ERM was identified as the upper edge of the RNFL in 15.2% to 36.1% of scans, falsely inflating the RNFL or macular thickness.32
Errors of automated segmentation of the retinal layers represent device-dependent errors and can result in misinterpretation. These errors are more prevalent in highly myopic eyes whose axial length may be outside of the reference database and may confound the machine when the anatomy is altered in tilted disc, staphyloma and retinoschisis cases. Use of ganglion cell analysis may be more successful in these cases as they can be less influenced by the anatomy.32
This becomes particularly challenging in advanced disease, as there is greater segmentation variability. Clinicians should remember that it is critical in all stages of glaucoma to confirm the appropriateness of the segmentation.
VF Interpretation Dos and Don’tsTo assess findings on the VF, in the context of already having OCT information, it is important to review fundamental “dos and don’ts” of VF interpretation. • DON’T discard the initial field. Just because it is the first field doesn’t mean it isn’t valid. • DO determine whether the field is reliable. Traditionally, only the reliability indices (fixation loss, false negative and false positive [FP]) were used to substantiate reliability, with FPs the most important of the three. FP percentages in excess of 15% are often associated with poor test results, and some practitioners require even more stringent standards.49 • DO compare the total deviation and pattern deviation. Total deviation numerical maps compare the sensitivity of individual test points to age-matched normals, and total deviation probability maps identify which of these points are abnormal. Mean deviation approximately represents the average of these total deviation values. Pattern deviation maps show localized VF loss after filtering out general depression or elevation. Thus, if the total deviation is significantly more depressed than the pattern deviation, there is likely a cataract. If the opposite is true (pattern deviation more depressed than total deviation), the patient is most likely trigger happy with high false positives; alternatively, they may have performed at a level exceeding the expectations for their age. • DON’T ignore the total deviation. Pattern deviation correction of diffuse changes in the visual field can paradoxically result in insufficiently assessing widespread damage from glaucoma.50 • DO interpret the global indices. This includes glaucoma hemifield test, visual field index, mean deviation and pattern standard deviation. It’s important to stage glaucoma accurately with the HPA classification.4 The HPA considers not only the extent of overall damage using mean deviation, but also the number of defective points in the pattern standard deviation probability map, as well as the proximity and density of loss near fixation.
• DO repeat tests to account for variability in VF change. The clinical significance of any visual field change usually depends on the number of exams given and the amount of intra-day and inter-visit variability of those exams. In general, peripheral test points not only vary more than central test points, but also exhibit progressively greater variability as the severity of the disease increases, with the variability peaking at -20dB on mean deviation and 8dB on pattern standard deviation.51,52 Additionally, patients with more global diffuse loss tend to have more variability than patients with localized loss.52 • DO acquire good baseline visual fields. These are essential for accurately detecting progression. The World Glaucoma Association recommends at least two reliable baseline VFs in the first six months of management and at least two additional fields over the next 18 months.53 More frequent visual fields may be necessary in advanced disease to detect fast progressors (-2dB/year or faster). The association recommends six VFs in the first two years in patients at risk for visual disability. • DON’T undertest. Undertesting reduces the ability to compensate for variability. Research shows that three examinations per year are required to detect a -2dB change in mean deviation (considered rapid progression) over a two-year period.54 It would take five years to confirm that same -2dB change if only two visual field exams are performed per year. • DON’T expect new defects to occur first as the disease progresses. Progression typically occurs through deepening and expansion of preexisting defects, not by the development of new defects.55 There can be substantial loss within the “black,” and failure to look at the numerical deviation can result in missing a deepening defect. • DO look for glaucomatous patterns of loss. The presence of classic patterns of visual field loss such as paracentral scotomas, nasal steps and arcuate defects shows that the patient has damage consistent with glaucoma.49 • DON’T, however, disregard depressed test points. This is true if a perfect traditional pattern of loss is not present. Glaucomatous VFs can exhibit a wide variety of patterns, so the lack of traditional defects should actually be expected in many of our patients.45 It is important to assess visual fields in the context of where local and diffuse damage is found topographically on RNFL and macular OCT scans. If there is alignment between the area of damage on the OCT and the region of VF that samples the damage, a classic pattern is not required to substantiate the visual field defect’s legitimacy. • DO consider switching from standard test strategies. This can help to better detect damage. Multiple studies show 24-2 VFs can miss or markedly underestimate defects near fixation, whereas 10-2 strategies can better detect these defects.43 Additionally, the use of 10-2 has been shown to correlate well with RGC thickness, improving the structure-function relationship.56 |
Choosing OCT Parameters
Glaucoma preferentially affects the GCC, which is comprised of the GCL, the macular RNFL and the IPL. Experimental models of glaucoma show substantial loss of RGCs in the parafoveal region. Given that approximately 50% of the RGCs are concentrated within that parafoveal region and that negligible population variability exists there, it is an ideal location to assess throughout the entire spectrum of glaucomatous disease using OCT.34
Each platform measures the GCC differently, including the ILP and GC-IPL, the entire GCC by total macular thickness and by isolation of the GCL. The strongest structure-function relationship has been correlated with isolated GCL, GC-IPL and the GCC.13 A weaker S-F relationship was observed with the full macular thickness parameters.
Let’s take a look at some of these imaging parameters.
Average GC-IPL thickness. Studies show average GC-IPL has excellent intra- and inter-visit reproducibility, and a repeatable 2µm or greater decrease in average GC-IPL is considered a statistically significant change.34 Additionally, because it removes macular RNFL from the segmentation, GC-IPL is less influenced by variability in that layer.35
Minimum GC-IPL thickness. This was designed to be sensitive to focal RGC loss and, not surprisingly, is consistently found across studies to be the most accurate GC-IPL parameter for the diagnosis of early, moderate and severe glaucoma.35,36 It’s followed in accuracy by inferotemporal and average GC-IPL thickness, respectively.
Tracking progression with minimum GC-IPL may be limited by its reproducibility. A change of 8µm to the average minimum GC-IPL over two exams is required to feel comfortable that the change is due to glaucomatous progression.34
In analyzing the rate of progression, no standard exists to designate a fast rate of average GC-IPL loss. For reference, researchers have reported the rate of average GC-IPL loss to be -0.014µm/year in healthy patients and -0.57µm/year in those with glaucoma.37
RNFL Thickness—Harbinger of ChangeRNFL thickness has been the single most commonly studied OCT parameter in glaucoma. A repeatable 4µm or greater decrease in global RNFL thickness on two consecutive scans compared with baseline is considered a statistically significant change.31 Research shows global/average cpRNFL thickness is the most reproducible RNFL parameter and helpful in distinguishing normal patients from those with glaucoma.36 Average RNFL, likely due to its excellent reproducibility, is also the best parameter to detect change in glaucoma patients across multiple platforms and should be the first parameter looked at on RNFL trend analysis.36 Although normal variability can confound things, patients with thinner RNFL averages are generally more likely to have glaucoma. Research has estimated the average rates of spectral domain OCT-based RNFL thinning in glaucoma patients ranges between -0.76µm and 1.5µm per year. At present, there is no universal standard as to what rate of RNFL progression is clinically important.48 More specific RNFL parameters, such as quadrants, sectors and clock hours are less reproducible than global averages, but still have excellent diagnostic value. Across all platforms, the best diagnostic regions for clinical use are the inferior and superior quadrants, with the inferior regions having more value than the superior regions.36 |
Inferior GC-IPL thickness. Research postulates the inferior macular ganglion cell layer is the earliest and most affected layer in glaucomatous changes.38 However, isolation of GCL is limited by both machine segmentation and the small range of tissue thickness that can be captured, making it less useable for progression analysis than GC-IPL.13 Segmentation becomes more challenging with advanced disease as thinner tissue layers become harder to find, increasing variability.12
Macular thickness asymmetry analysis. Comparison of tissue symmetry is another helpful diagnostic technique. Studies show inter-eye macular asymmetry analysis using TMT, termed posterior pole asymmetry analysis (PPAA), has equal, if not superior, diagnostic performance to circumpapillary RNFL (cpRNFL).39-41 Research also shows intra-eye GC-IPL asymmetry can help diagnose glaucoma in highly myopic eyes, where RNFL scans may be limited due to optic nerve head anatomy.42
Ultimately, numerous studies have compared the sensitivity of macular, RNFL and optic parameters, finding similar diagnostic performance.36 As a result, combining the data from all these parameters would likely provide better diagnostic value. One study did, in fact, find that combined analysis of GC-IPL and cpRNFL performed better diagnostically than the individual parameters by themselves.14 The investigators recently validated the performance of this combined index, known as the University of North Carolina’s UNC OCT Index, showing that it is likely a better tool for early detection than using individual parameters.43
Incorporating VF and OCT
Acknowledging that the structure-function relationship is complicated, how should we approach cases where VF and OCT results are inconsistent? First, by expecting the inconsistency, as we know structure and function rarely capture change at the same time. Given this expectation, a practical approach is to find the best strategy to detect change in the individual patient. Once glaucoma is diagnosed clinically, either VF or OCT may emerge as the most precise technology with which to gauge progression in that specific patient—and it generally depends on the stage of the disease.8 For instance, an early glaucoma case that shows no 24-2 VF defect, yet shows glaucomatous loss of RNFL and GC-IPL, may benefit from greater reliance on tracking those OCT parameters. In that same scenario, changing the VF test strategy or stimulus size might increase the likelihood of S-F alignment and decrease perceived inconsistencies.44
If these testing modifications are undertaken and fail to elicit improved consistency, greater analytical emphasis would be placed on OCT for that patient. However, functional testing would not be abandoned here, as the point at which RGC dysfunction will be captured by VF testing in this scenario is unknown. Conversely, if a repeatable glaucomatous visual field defect precedes expected thinning on OCT, it would not result in abandoning the use of OCT testing.
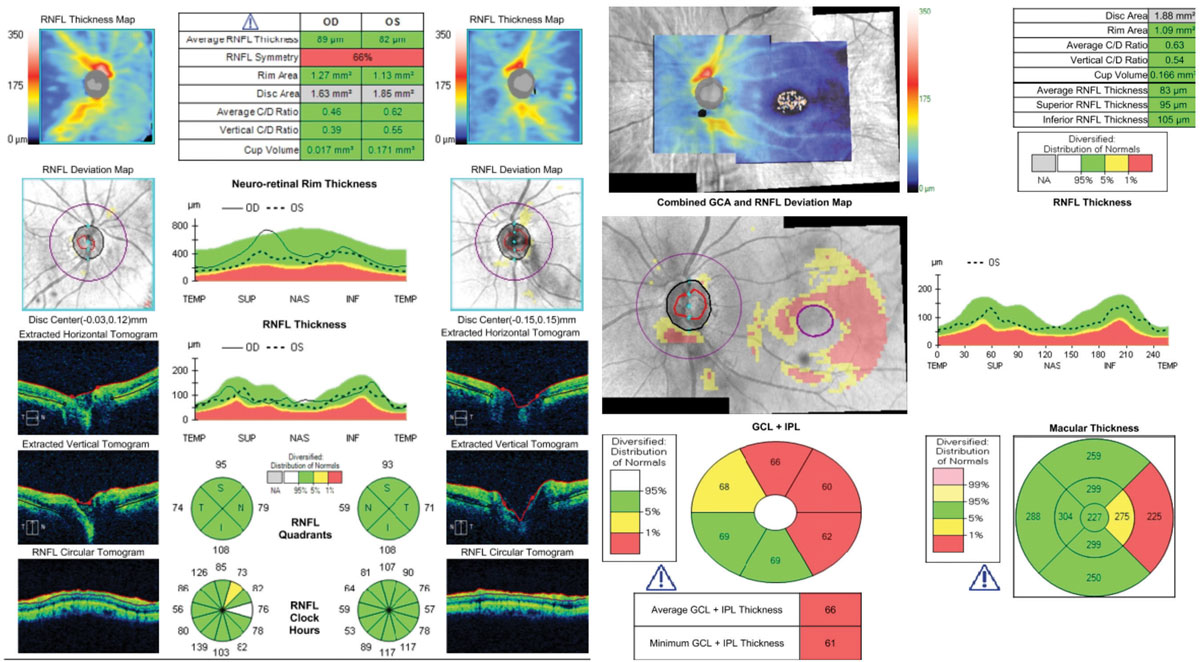 |
| This 62-year-old patient shows optic discs that appear glaucomatous OS>>OD funduscopically. Cursory analysis of the RNFL printout (at left) alone might give the false sense that there are no pathological changes compared with the reference database. The additional analysis of all tissues affected by glaucoma, at right, confirms the diagnosis of early glaucoma. This highlights the necessity of using all of the information these machines provide. Click image to enlarge. |
The best strategy is to incorporate VF and OCT as complementary tests for the diagnosis and management of glaucoma. A recent editorial advocated discontinuing the debate about whether and when clinicians should use one form of testing over the other.24 The authors pointed out that if either method was optimal, there would be no need for the other. Ideally, we would more effectively use the information gleaned from both technologies. Efficiently integrating VF, RNFL tissue segmentation and thickness maps, as well as RGC data in a meaningful way would allow us to maximize how we use that information.
Currently, the “Hood Report” (available on Topcon and Heidelberg platforms) is the only commercially available software to accomplish this. The Hood Report incorporates VF points overlaid by RNFL and either GCL+ (Topcon) or GCL (Heidelberg) thickness and significance maps produced from a single widefield cube scan. Research shows this single-page diagnostic report performs as well or better in classifying an eye as glaucomatous than that of glaucoma subspecialists who had fundus photos, 24-2 VFs and widely available commercial OCT RNFL.45
Looking to the future, integration of structural and functional data into one metric may streamline diagnosis, staging and determination of rate of change. One proposed metric is a combined S-F index (CSFI), based on experimental estimates of the percentage of RGCs lost compared with that expected for an age-matched healthy eye. Multiple studies show the CSFI successfully assesses rates of change throughout the entire spectrum of disease, unlike with isolated structural or functional testing.46 Newer technologies will likely individualize metrics in the future by estimating the quantity of RGCs in real time.47
Ultimately, in 2019 we must accept that both structural and functional testing have advantages and disadvantages, and neither are optimal. Their complementary use gives us the best opportunity to detect progression in our patients, which is key to preserving their quality of life.
Dr. Ristin is an attending optometrist at the Jesse Brown Veterans Affairs Medical Center (VAMC) in Chicago. She is the immediate past chair of the Glaucoma Section of the American Academy of Optometry (AAO).
Dr. Rixon is the residency coordinator at the Memphis VAMC, a member of the Optometric Glaucoma Society and is pursuing his glaucoma diplomate through the AAO Glaucoma Section.
| 1. Nouri-Mahdavi K. Selecting visual field tests and assessing visual field deterioration in glaucoma. Can J Ophthalmol. 2014;49:497-505. 2. Lamparter J, Russell RA, Schulze A, et al. Structure-function relationship between FDF, FDT, SAP, and scanning laser ophthalmoscopy in glaucoma patients. Invest Ophthalmol Vis Sci. 2012;53:7553-59. 3. Camp AS, Weinreb RN. Will perimetry be performed to monitor glaucoma in 2025? Ophthalmology. 2017;124(12S):S71-S75. 4. Hodapp E, Parrish RK II, Anderson DR. Clinical Decisions in Glaucoma. St Louis: CV Mosby Co; 1993: 52-61. 5. Malik R, Swanson WH, Nicolela MT. Structure-function relationships in glaucoma. In: Shaarawy T, Sherwood MB, Hitchings RA, Crowston JG. Glaucoma. London: Elsevier Saunders; 2015. 6. Lucy KA, Wollstein G. Structural and functional evaluations for the early detection of glaucoma. Expert Rev Ophthalmol. 2016;11(5):367-76. 7. Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53(11):6939-46. 8. Zhang X, Dastiridou A, Francis BA, et al. Comparison of glaucoma progression detection by optical coherence tomography and visual field. Am J Ophthalmol. 2017;184:63-74. 9. Kuang TM, Zhang C, Zangwill LM, et al. Estimating lead time gained by optical coherence tomography in detecting glaucoma before development of visual field defects. Ophthalmology. 2015;122(10):2002-09. 10. Abe RY, Diniz-Filho A, Zangwill LM, et al. The relative odds of progressing by structural and functional tests in glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):421-428. 11. Bowd C, Zangwill LM, Weinreb RN, et al. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. Am J Ophthalmol. 2017;175:37-44. 12. Belghith A, Medeiros FA, Bowd C, et al. Structural change can be detected in advanced-glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):511-8. 13. Miraftabi A, Amini N, Morales E, et al. Macular SD-OCT outcome measures: Comparison of local structure-function relationships and dynamic range. Invest Ophthalmol Vis Sci. 2016;57(11):4815-23. 14. Mwanza J-C, Budenz DL, Godfrey DG, et al. Diagnostic performance of optical coherence tomography ganglion cell-inner plexiform layer thickness measurements in early glaucoma. Ophthalmology. 2014;121(4):849-54. 15. Kim Ek, Park HL, Park CK. Segmented inner plexiform layer thickness as a potential biomarker to evaluate open-angle glaucoma: dendritic degeneration of retinal ganglion cell. PLoS One. 2017;12(8):e0182404. 16. Lin JP, Lin PW, Lai IC, Tsai JC. Segmental inner macular layer analysis with spectral-domain optical coherence tomography for early detection of normal tension glaucoma. PLoS One. 2019;14(1):e0210215. 17. Malik R, Swanson WH, Garway-Heath DF. ‘Structure-function relationship’ in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol. 2012;40(4):369-80. 18. Phu J, Khuu SK, Yapp M, et al. The value of visual field testing in the era of advanced imaging: clinical and psychophysical perspectives. Clin Exp Optom. 2017;100(4):313-32. 19. Tatham AJ, Weinreb RN, Medeiros FA. Strategies for improving early detection of glaucoma: the combined structure-function index. Clin Ophthalmol. 2014 Mar 26;8:611-21. 20. Susanna R, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1. 21. Garway-Heath DF, Quartilho A, Prah P, et al. Evaluation of visual field and imaging outcomes for glaucoma clinical trials (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2017 Aug;115:T4. 22. Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96(1):47-52. 23. Alasil T, Wang K, Yu F, et al. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol. 2014;157(5):953-59. 24. Medeiros FA, Tatham AJ. Structure versus function in glaucoma: The debate that doesn’t need to be. Ophthalmology. 2016;123(6):1170-72. 25. Öhnell H, Heijl A, Brenner L, et al. Structural and functional progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2016;123(6):1173-80. 26. Mwanza J-C, Budenz DL. Optical coherence tomography platforms and parameters for glaucoma diagnosis and progression. Curr Opin Ophthalmol. 2016;27(2):102-10. 27. Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119(9):1858-66. 28. Lee ES, Kang SY, Choi EH, et al. Comparisons of nerve fiber layer thickness measurements between Stratus, Cirrus, and RTVue OCTs in healthy and glaucomatous eyes. Optom Vis Sci. 2011;88(6):751-58. 29. Wu Z, Saunders LJ, Zangwill LM, et al. Impact of normal aging and progression definitions on the specificity of detecting retinal nerve fiber layer thinning. Am J Ophthalmol. 2017;181:106-13. 30. Silverman AL, Hammel N, Khachatryan N, et al. Diagnostic accuracy of the Spectralis and Cirrus reference database in differentiating between healthy and early glaucoma eyes. Ophthalmology. 2016;123(2):408-14. 31. Sayed MS, Margolis M, Lee RK. Green disease in optical coherence tomography diagnosis of glaucoma. Curr Opin Ophthalmol. 2017;28(2):139-53. 32. Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014;132(4):396-402. 33. Hardin JS, Taibbi G, Nelson SC, et al. Factors affecting Cirrus-HD OCT optic disc scan quality: a review with case examples. J Ophthalmol. 2015;2015:746150. 34. Mwanza J-C, Oakley JD, Budenz DL, et al. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(11):8323-29. 35. Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(7):4422-29. 36. Chen TC, Hoguet A, Junk AK, et al. Spectral-domain OCT: helping the clinician diagnose glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(11):1817-27. 37. Hammel N, Belghith A, Weinreb RN, et al. Comparing the rates of retinal nerve fiber layer and ganglion cell-inner plexiform layer loss in healthy eyes and in glaucoma eyes. Am J Ophthalmol. 2017;178:38-50. 38. Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013 Jan;32:1-21. 39. Sullivan-Mee M, Ruegg CC, Pensyl D, et al. Diagnostic precision of retinal nerve fiber layer and macular thickness asymmetry parameters for identifying early primary open-angle glaucoma. Am J Ophthalmol. 2013;156(3):567-577.e1. 40. Dave P, Shah J. Diagnostic accuracy of posterior pole asymmetry analysis parameters of Spectralis optical coherence tomography in detecting early unilateral glaucoma. Indian J Ophthalmol. 2015;63(11):837-42. 41. Mori S, Hangai M, Sakamoto A, Yoshimura N. Spectral-domain optical coherence tomography measurement of macular volume for diagnosing glaucoma. J Glaucoma. 2010;19(8):528-34. 42. Kim YK, Yoo BW, Jeoung JW, et al. Glaucoma-diagnostic ability of ganglion cell-inner plexiform layer thickness difference across temporal raphe in highly myopic eyes. Invest Ophthalmol Vis Sci. 2016;57(14):5856-63. 43. Mwanza J-C, Lee G, Budenz DL, et al. Validation of the UNC OCT Index for the diagnosis of early glaucoma. Transl Vis Sci Technol. 2018;7(2):16. 44. Hood DC, De Moraes CG. Four questions for every clinician diagnosing and monitoring glaucoma. J Glaucoma. 2018;27(8):657-64. 45. Hood DC. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res. 2017;57:46-75. 46. Zhang C, Tatham AJ, Daga FB, et al. Event-based analysis of visual field change can miss fast glaucoma progression detected by a combined structure and function index. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1227-34. 47. Raza AS, Hood DC. Evaluation of the structure-function relationship in glaucoma using a novel method for estimating the number of retinal ganglion cells in the human retina. Invest Ophthalmol Vis Sci. 2015;56(9):5548-56. 48. Saunders LJ, Medeiros FA, Weinreb RN, Zangwill LM. What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol. 2016;11(3):227-34. 49. Heijl A, Patella VM, Bengtsson B. The Field Analyzer Primer: Effective Perimetry. 4th ed. Dublin, CA; Carl Zeiss Meditec: 2012. 50. Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128(12):1528-32. 51. De Moraes CG, Liebmann JM, Levin LA. Detection and measurement of clinically meaningful visual field progression in clinical trials for glaucoma. Prog Retin Eye Res. 2017 Jan;56:107-47. 52. Russell RA, Garway-Heath DF, Crabb DP. New insights into measurement variability in glaucomatous visual fields from computer modelling. PloS One. 2013;8(12):e83595. 53. Weinreb RN. Progression of Glaucoma: the 8th Consensus Report of the World Glaucoma Association. Amsterdam: Kugler Publication; 2011. 54. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569-73. 55. Nouri-Mahdavi K, Nassiri N, Giangiacomo A, Caprioli J. Detection of visual field progression in glaucoma with standard achromatic perimetry: a review and practical implications. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1593-616. 56. Lee J-W, Morales E, Sharifipour F, et al. The relationship between central visual field sensitivity and macular ganglion cell/inner plexiform layer thickness in glaucoma. Br J Ophthalmol. 2017;101(8):1052-58. 57. Katz J, Sommer A. Reliability indexes of automated perimetric tests. Arch Ophthalmol. 1988;106(9):1252-4. 58. Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000;41(8):2201-4. 59. Ishiyama Y, Murata H, Mayama C, Asaoka R. An objective evaluation of gaze tracking in Humphrey perimetry and the relation with the reproducibility of visual fields: a pilot study in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(12):8149-52. 60. Ishiyama Y, Murata H, Asaoka R. The usefulness of gaze tracking as an index of visual field reliability in glaucoma patients. Invest Ophthalmol Vis Sci. 2015;56(11):6233-36. |
