 |
Intraocular malignant neoplasm is one of the most feared diagnoses an OD may have to make. The visual prognosis of this ocular cancer is usually poor, and the potential for systemic involvement is worrisome. However, what may at first glance appear to fit the bill may be an imposter syndrome. Making the correct assessment before sending the patient out for further testing is imperative to distinguish intraocular malignant neoplasms from these other disorders that only masquerade as a malignancy.
Here, we review the case of a 69-year-old Caucasian male who was ultimately diagnosed with sclerochoroidal calcification (SCC). This condition is correctly diagnosed and referred by only 5% of practitioners.1 Here’s how to be among them and avoid superfluous testing and patient anxiety.
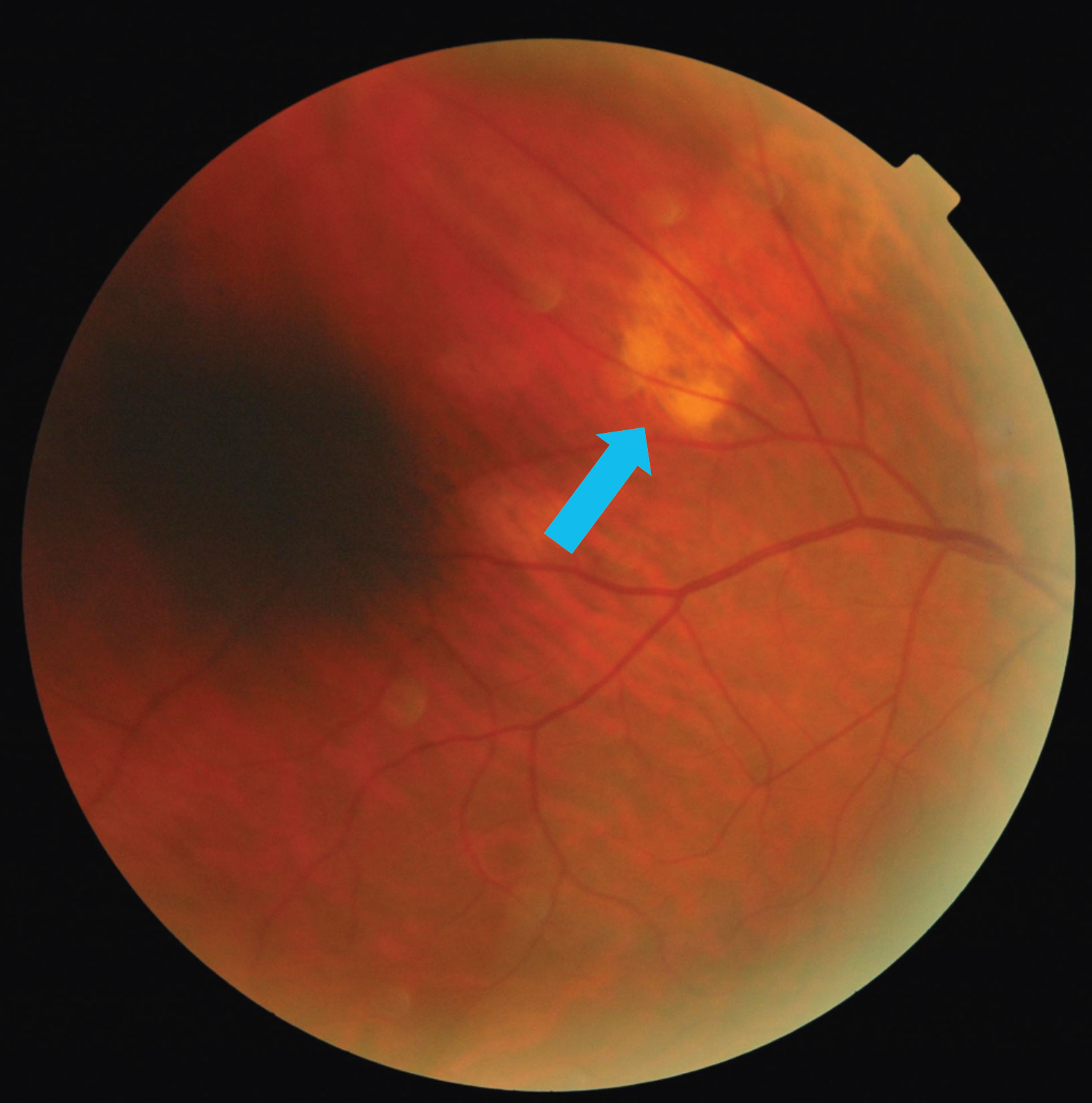 |
| Fig. 1. The blue arrow in this fundus photo points to a subretinal lesion in the right eye’s superior temporal quadrant. Click image to enlarge. |
Examination
Our patient presented for follow-up of mild-stage primary open-angle glaucoma, age-related nuclear cataracts and an area of retinal pigment epithelium (RPE) dropout superiorly in the right eye. His medical history included Type 2 diabetes, essential hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, benign prostatic hyperplasia and obstructive sleep apnea. His systemic and ocular medications included Lipitor (atorvastatin, Pfizer) carvedilol, insulin, Vyzulta (latanoprost, Bausch + Lomb), Cozaar (losartan, Merck), metformin and Flomax (tamsulosin, Boehringer Ingelheim). His social history was positive for tobacco (half a pack of cigarettes per day) and recreational marijuana use.
His best-correct visual acuities were 20/20 OD, OS with +0.75-0.50x120 OD and +0.25-0.50x115 OS and a +2.50 add. Chair skills were unremarkable. Slit lamp biomicroscopy showed a pinguecula OU and NC2 OU as graded by the lens opacities classification system (LOCS) III. A dilated fundus examination revealed a cup-to-disc ratio of 0.70V/0.65H OD and 0.50 OS, along with a yellow subretinal lesion outside the superior temporal arcade (Figure 1).
Enhanced-depth imaging optical coherence tomography (EDI-OCT) through the area demonstrated choroidal thinning above the lesion (Figure 2). B-scan ultrasonography highlighted an echo-dense placoid mass with acoustic orbital shadowing (Figure 3).
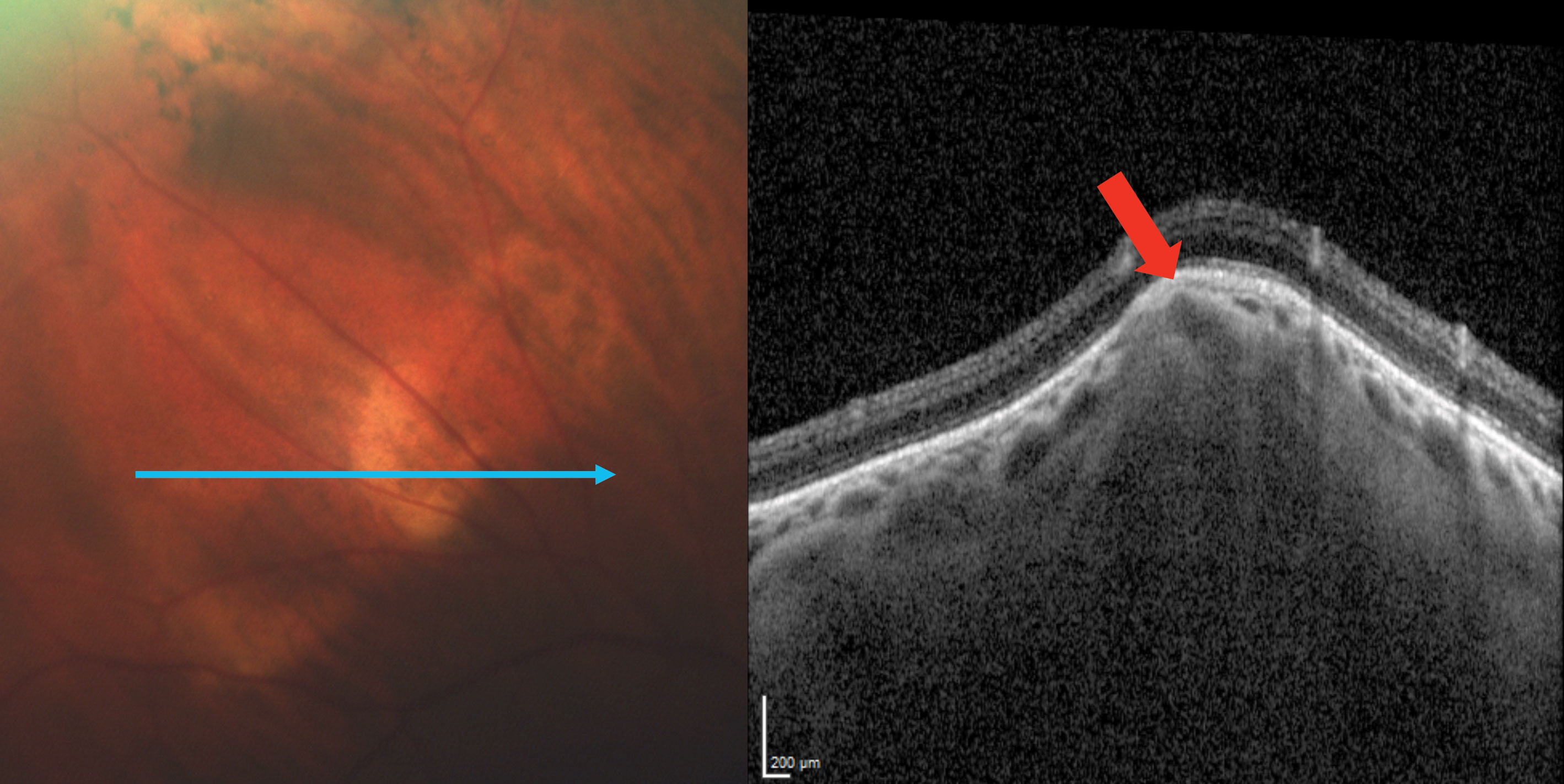 |
| Fig. 2. EDI-OCT imaging through the SCC reveals a Type 2 “rolling” lesion. The red arrow points to an area of choroidal thinning above the lesion. Click image to enlarge. |
Discussion
SCC is a deposition of calcium within the sclera. They present as single or multiple, round focal lesions along the superior temporal arcades and are most commonly seen in the elderly as an incidental finding.2-4 SCCs appear pale yellow, white or orange and can be flat or up to 6mm in height.5 A 2015 study analyzed 179 eyes of 118 patients with SCC and found the vast majority in Caucasians (98%) with a mean age of 69 and a 60:40 female-to-male ratio.1 Laterality approached 50% for all categories: unilateral vs. bilateral, and right eye vs. left eye.1
The term SCC is actually a misnomer, as the calcification represents a scleral mass. There are four recognized types: Type 1 “flat,” Type 2 “rolling,” Type 3 “rocky-rolling” and Type 4 “table mountain.”6 “Rocky-rolling” represents the most common type and causes the most thinning of the overlying choroid, which in turn leads to a greater possibility for adjacent retinal pigment epithelium (RPE) and outer retinal changes (Figure 4).6 The hill-like elevation in this patient characterizes a Type 2 “rolling” SCC (Figure 2).
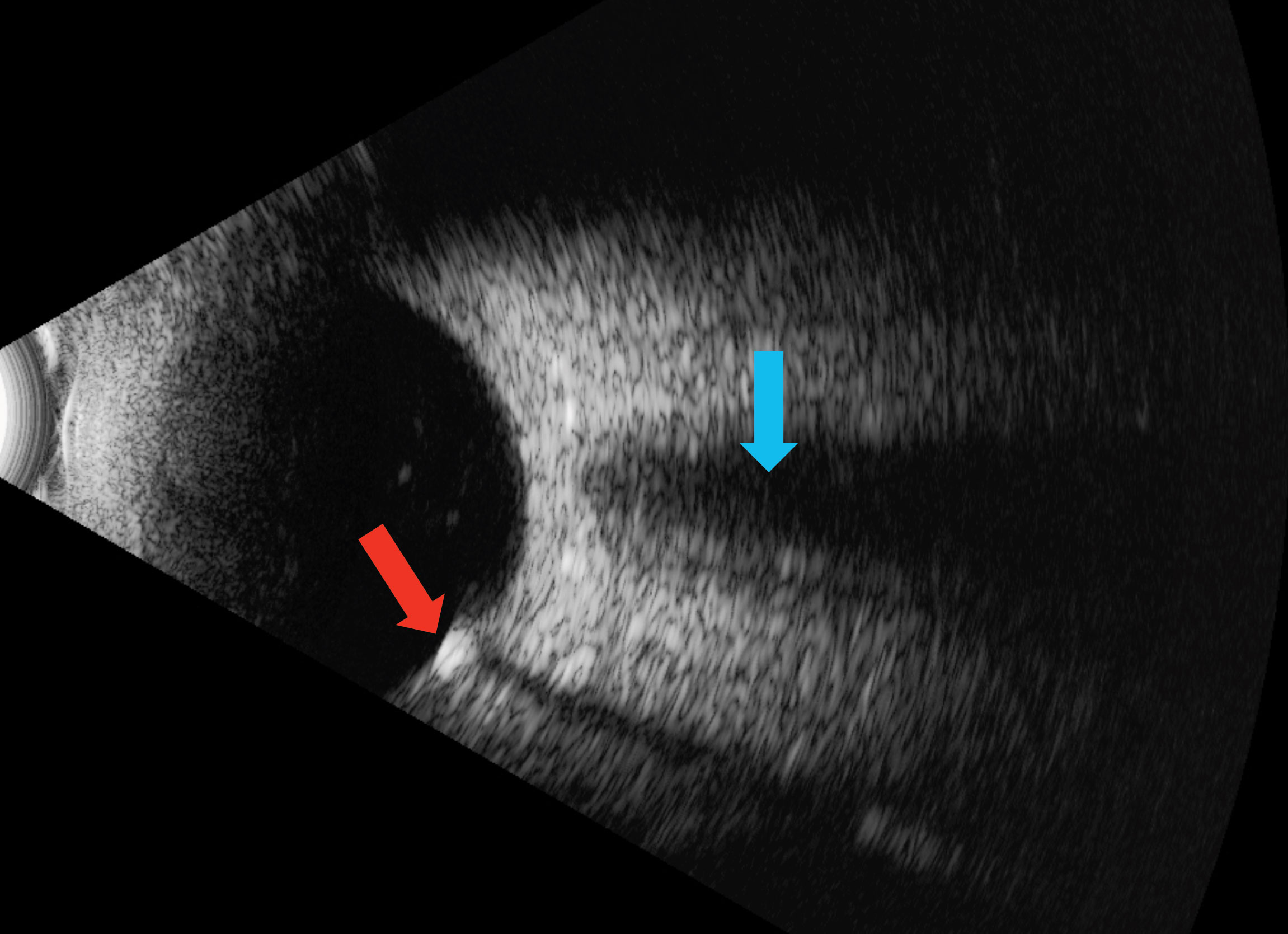 |
| Fig. 3. The red arrow shows an echo-dense placoid lesion while, the blue shows acoustic orbital shadowing on this B-scan ultrasound. Click image to enlarge. |
Making the Call
Differentiating SCC from more insidious lesions is key to preventing superfluous testing. Funduscopy alone can diagnose SCC, given its characteristic appearance, demographic, and predilection for the superior temporal location. With EDI-OCT, an SCC will appear as a scleral (not choroidal) plaque with secondary compression of the choroid.3,7 B-scan ultrasonography shows a highly-reflective, echo-dense plaque unlike a choroidal metastasis, which would likely exhibit low-to-medium reflectivity. The majority of SCCs exhibit homogeneous hyperautofluorescence with fundus autofluorescence, and hyperreflectivity on infrared imaging.7 SCCs also lack vasculature on a fluorescein or indocyanine green angiography. On computed tomography (CT) scans, SCCs appear as highly-attenuated objects similar to bone. The CT scan easily confirmed the right eye SCC in this patient, and surprisingly picked up a subtle one in the left eye, which was not viewed clinically (Figure 5). This likely represents a Type 1 “flat” lesion, which is detectable only with ultrasonography or CT exam.1
Approximately 80% of SCCs are primary or idiopathic.1,6 Secondary causes result from hypercalcemia due to hyperparathyroidism, parathyroid adenomas, hypervitaminosis D, calcium pyrophosphate dihydrate deposition, hypomagnesemia, diuretic use, chronic kidney disease and, rarely, renal disorders, including Bartter’s syndrome.2,5,8
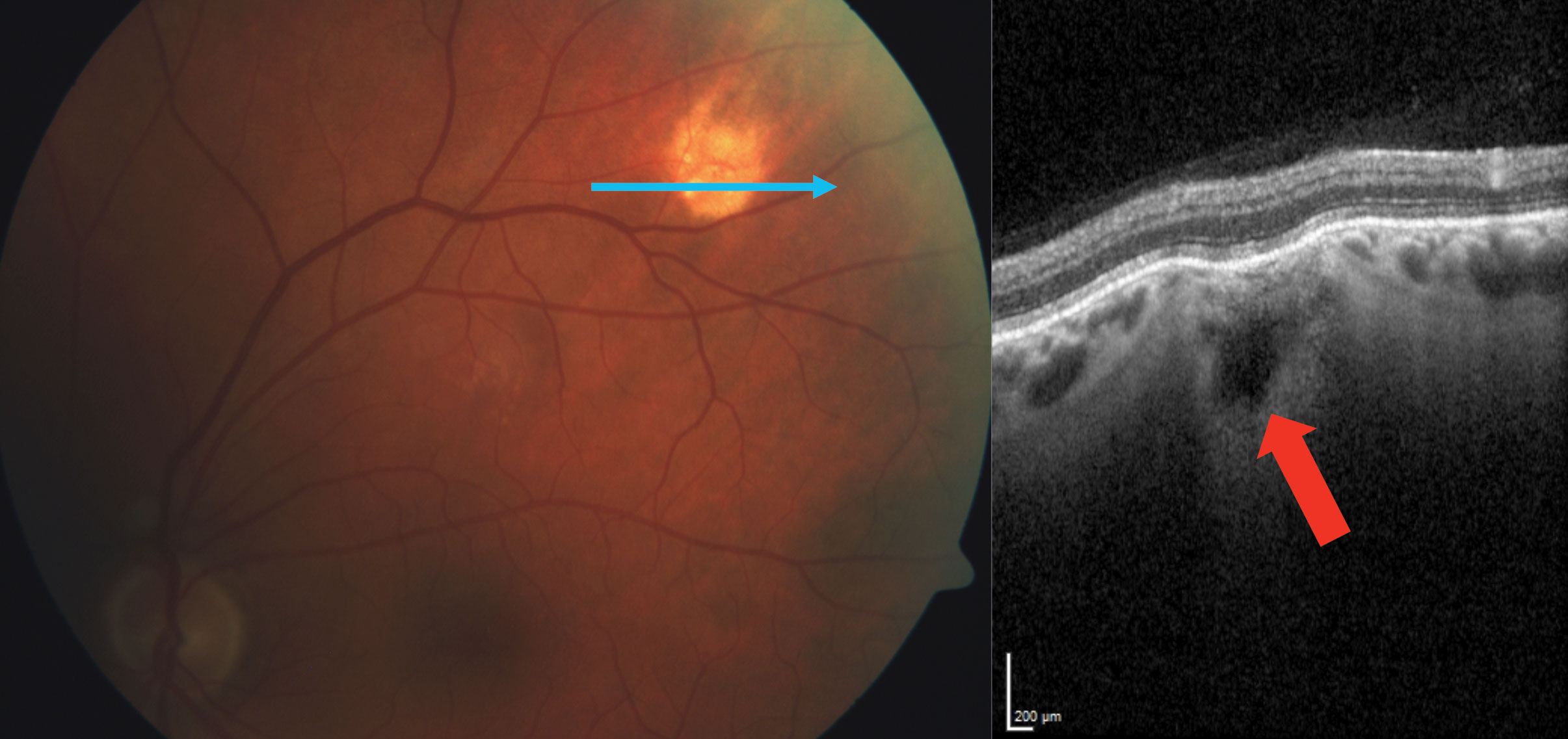 |
| Fig. 4. Another SCC patient exhibits a Type 3 “rocky-rolling” appearance. The red arrow shows the extensive choroidal thinning above the scleral mass. Click image to enlarge. |
Patients with hyperparathyroidism display the mnemonic “stones, bones, groans, thrones and psychiatric overtones” due to kidney stones, bone pain, abdominal pain, polyuria and psychiatric symptoms, such as depression, anxiety, insomnia, cognitive dysfunction and possibly coma.9
Bartter’s syndrome represents a group of inherited primary renal tubular electrolyte transport disorders. Of the three phenotypes, only two—classic Bartter’s and Gitelman’s—are associated with SCC.8 Both of these closely-related autosomal recessive diseases share the characteristics of renal salt wasting and hypokalemic hypochloremic metabolic alkalosis.10 A manifestation of Gitelman’s syndrome—which is more common and presents later in childhood or adulthood—includes hypomagnesemia.11 Magnesium ions increase calcium pyrophosphate dihydrate crystal solubility. Therefore, hypomagnesemia could lead to deposition of those calcium crystals in joints and the sclera.11
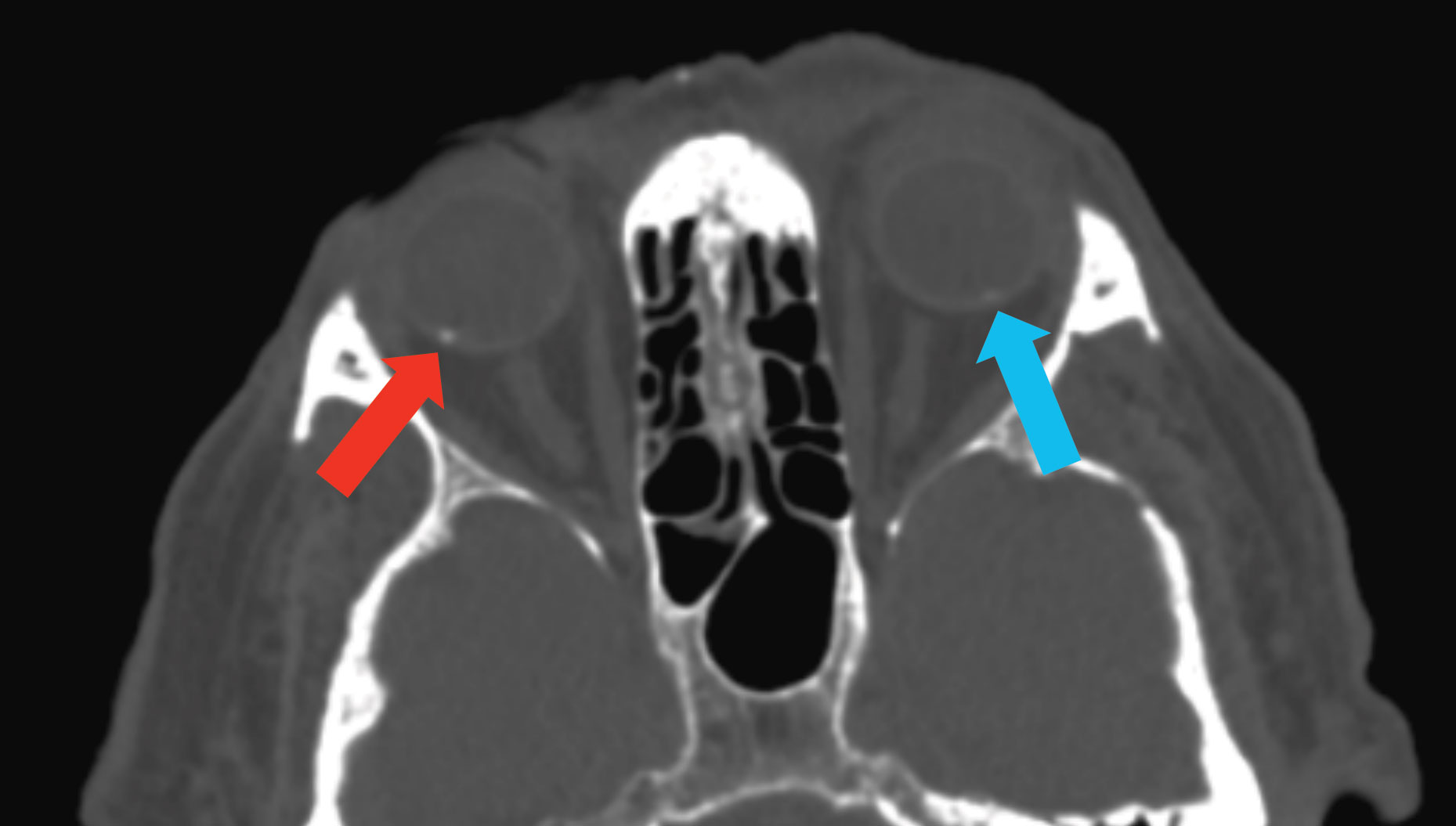 |
| Fig. 5. This CT scan demonstrates SCC in the right eye (blue arrow) and a subtle one in the left eye (red arrow), which was not visible on clinical exam. Click image to enlarge. |
SCCs are usually benign and are similar to a Cogan or senile scleral-calcified plaque, which occurs near the horizontal rectus muscle insertions. Some researchers speculate that SCCs may be their counterparts at the oblique muscle insertions where chronic forces induce both primary and secondary calcifications.5 Rarely, SCCs may lead to a secondary choroidal neovascular membrane (CNV). Researchers recommend periodically monitoring SCC patients for CNV development with a dilated exam and EDI-OCT.2,9 If a CNV presents, observation may be the best initial course of action, given the more peripheral location to the macula.12 Intravitreal anti-vascular endothelial growth factor injections or argon laser may also be approriate.12
SCCs can impersonate various intraocular tumors and are frequently misdiagnosed.2,5,9 Typically, they represent an idiopathic incidental finding, but their discovery does warrant appropriate systemic testing to rule out underlying conditions. This may include a complete metabolic panel, parathyroid and renal function testing.7 Medications should also be reviewed as diuretics can lead to a loss of metabolic parameters.1 More importantly, however, is making the right diagnosis and avoiding unnecessary testing.
Dr. Williamson is the residency supervisor at the Memphis VA Medical Center and is an adjunct faculty at multiple optometry schools.
Dr. Kirk is a resident at the Memphis VA medical center.
| 1. Shields CL, Hasanreisoglu M, Saktanasate J, et al. Sclerochroidal calcification: clinical features, outcomes, and relationship with hypercalcemia and parathyroid adenoma in 179 eyes. Retina. 2015;35:547-54. 2. Ali ZC, David VP. Sclerochoroidal calcification associated with hypovitaminosis D. Can J Ophthalmol. 2017;52(4):e121-2. 3. Honavar S, Shields C, Demirci H, Shields J. Sclerochoroidal calcification: Clinical manifestations and systemic associations. Arch Ophthalmol. 2001;119:833-40. 4. Leys A, Stalmans P, Blanckaert J. Sclerochoroidal calcification with choroidal neovascularization. Arch Ophthalmol. 2000;118:854-7. 5. Wong C, Kawasaki B. Idiopathic sclerochoroidal calcification. Optom Vis Sci. 2014;91(2):32-7. 6. Hasanreisoglu M, Saktanasate J, Shields P, Shields C. Classification of sclerochoroidal calcification based on enhanced depth imaging optical coherence tomography “mountain-like” features. Retina. 2015;35:1407-14. 7. Fung A, Arias J, Shields C, Shields J. Sclerochoroidal calcification is primarily a scleral condition based on enhanced depth imaging optical coherence tomography. JAMA Ophthalmol. 2013;131(7):960-3. 8. Goerlitz-Jessen M, Ali M, Grewal D. Rare complications of sclerochoroidal calcifications. JAMA Ophthalmol. 2019;137(1):111-2. 9. Sugarman J, Douglass A, Say E, Shields C. Stones, bones, groans, thrones and psychiatric overtones: Systemic associations of sclerochoroidal calcification. Oman J Ophthalmol. 2017;10(1):47-9. 10. Fulchiero R, Seo-Mayer P. Bartter Syndrome and Gitelman Syndrome. Pediatr Clin North Am. 2019;66(1):121-34. 11. Bourcier T, Blain P, Massin P, et al. Sclerochoroidal calcification associated with Gitelman syndrome. Am J Ophthalmol. 1999;128(6):767-8. 12. Bessette AP, Singh AD. Multimodal imaging of choroidal neovascularization associated with sclerochoroidal calcification. Ocul Oncol Pathol. 2016;2(4):234-8. |

