Contact Lens Close-upThe August issue of Review of Optometry is our 47th annual contact lens report, where we help you use new and old techniques to improve your fitting skills. |
On a recent commute home, I listened with a distracted ear to a science podcast, and my interest grew as the columnist asked a simple question: What would we be without water? My thoughts then spiraled into the concrete world of my professional life. Extrapolating the question to eye care, I pondered: What would contact lenses be without water?
We all remember learning in optometry school that the first lenses were made of glass with no water content, then eventually of rigid polymer with a water content of no more than 1%.1 The physiological limitations of these materials and the difficulty of wearing them due to discomfort limited their use. In 1961, Otto Wichterle succeeded in developing a softer, more comfortable hydrophilic material, as well as a reliable centrifugal manufacturing process.2 As a chemist working on an artificial mandible, he had realized that material had optical properties and that the presence of water in a polymer provides a degree of flexibility and comfort, while promoting the passage of oxygen and other gases through its matrix, improving ocular physiological response to lens wear.
Voilà, the soft contact lens era began and it became a mainstream hit.
Because current-generation contact lenses are so advanced and relatively foolproof, we practitioners may have lost some awareness of their inner workings and how that affects clinical performance—so, let’s revisit the materials science fundamentals that make these products tick!
Contact Lens Material
A polymer is a large macromolecule composed of several series of repeating molecules called monomers. Every monomer has a covalent bond between each connecting unit.3 The polymer is composed of both hydrophilic and hydrophobic groups. The hydration of this polymer occurs as a result of the physicochemical equilibrium established between the water molecules and these two groups.4 More specifically, the water molecule can either be bound to a hydrophilic group, which retains it, or be trapped in a space formed within the polymer matrix. The physical properties of the material developed are governed by the state of the water molecules trapped or bound inside.5
Contact lenses often have tight bonds, meaning there is a direct hydrogen bond between the water and the polar groups of the matrix. Water molecules can also be attached with looser bonds, especially when the water molecules remain trapped in the small spaces in the matrix.
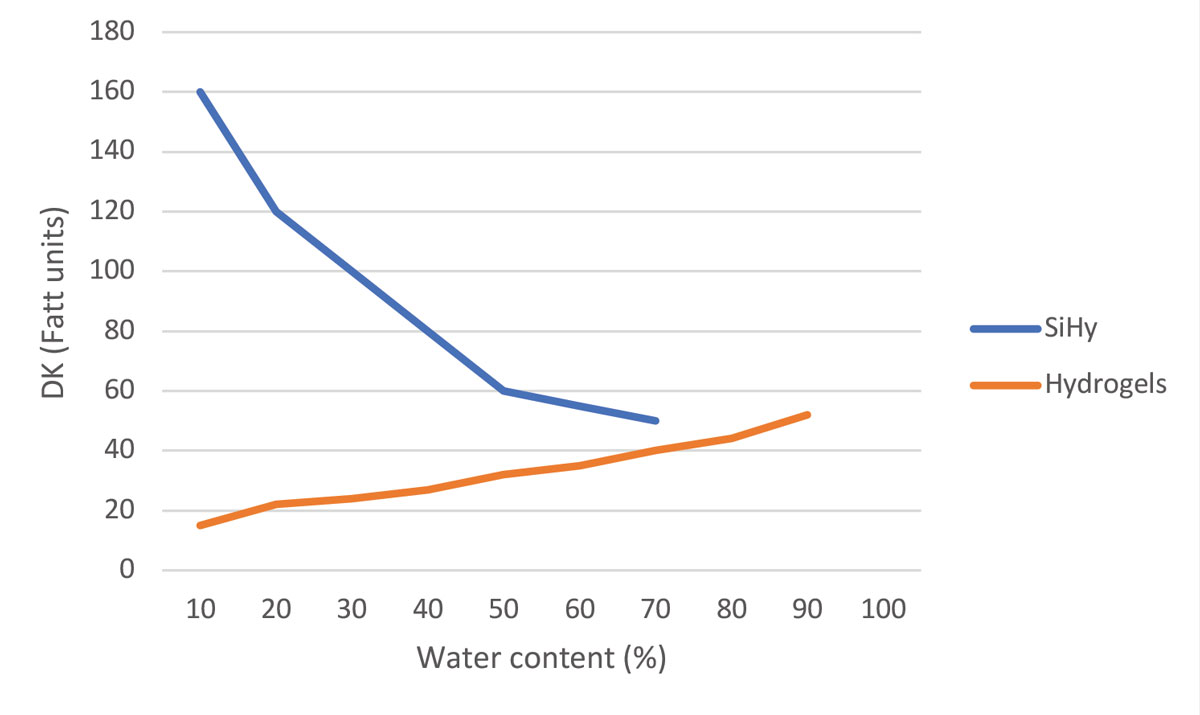 |
| Fig. 1. Graph of DK values (oxygen permeability) of silicone hydrogel and hydrogel lenses vs. their water content. Click image to enlarge. |
The rate of hydration, or the ability of a polymer to absorb water, depends on a number of factors: the nature of the hydrophilic groups, the density of crosslinks in the matrix, the rate of water saturation, the storage (or wear) environment of the polymer and the ambient temperature.4 Contrary to what one might intuitively think, the rate of absorption does not correlate with the matrix’s ability to generate tight bonds to water molecules. The rate of contact lens material hydration should be known by its ratio of tight bonds to free molecules, although this information is not provided by manufacturers. This ratio will increase in favor of tightly bound molecules in the presence of a larger network of crosslinks (as in the case of the HEMA polymer), since there are fewer spaces in which molecules can move freely.6
The presence of salts and electrolytes in a lens matrix, such as when it comes in contact with tears, increases the attachment sites of tight bonds and reduces the number of free water molecules in the matrix. Conversely, due to the limited number of tight bonds the matrix can form, as the water content of the polymer increases, the ratio decreases. However, maintaining a minimum number of free water molecules is essential because it is through these molecules—not the tightly bound ones—that the exchange of gases, including oxygen, can take place.7
Once applied to the eye, the lens is subject to body temperature, tear exchange, blinking forces and the environmental conditions affecting the tear film, such as osmolarity, pH and other properties. These factors cause the hydration level of the lens to vary throughout the day.
Free water molecules evaporate first, followed by molecules that are more tightly bound to the matrix. Therefore, it becomes clear that materials with a low level of bound molecules, and therefore more free molecules in the matrix, will tend to dehydrate more quickly.8
It’s important to understand that it’s not the percentage of water per se but the nature of the bond between the water molecules and the matrix that determines the rate of water loss from the polymer. For example, a lens with 40% water content may dehydrate faster than a lens with 75% water content, especially if the former has a higher proportion of free water molecules. In the end, a dehydrated lens will be more uncomfortable and will perform less well optically than a fully hydrated lens.
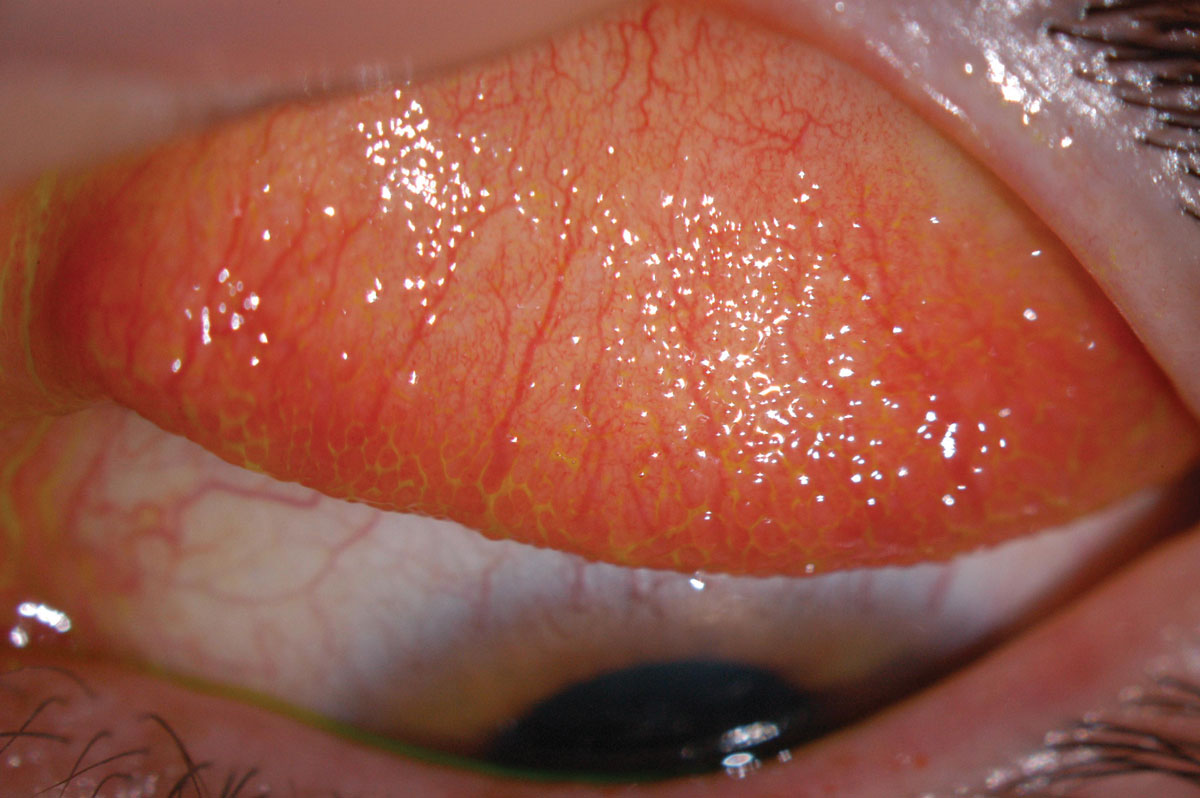 |
Fig. 2. Inflamed superior conjunctiva with papillary reaction (GPC). Click image to enlarge. |
HEMA vs. Silicone Hydrogel
All of the above applies primarily to HEMA (hydroxyethyl methacrylate) soft lenses manufactured and prescribed primarily between 1970 and 1990. HEMA is a polymer whose water content could vary from 30% to 80%, depending on what was desired.
A higher water content HEMA lens brings more oxygen to the eye and allows for better elimination of carbon dioxide produced by the normal metabolism of corneal cells. In the absence of adequate exchange, hypoxia or hypercapnia can occur, resulting in corneal acidosis, characterized in particular by a reduced cellular metabolism and rate of mitosis, loss of corneal transparency and the appearance of neovascularization from the limbus.9 The accumulation of microcysts in the cornea reflects a chronic lack of gas exchange over several weeks.10
HEMA lenses with a higher water content, however, are more difficult to handle and more prone to breakage. Lenses with higher water content were therefore reserved for extended wear. Based on very short-term trials, manufacturers advocated an escalation to longer and longer wearing periods without lens removal, ranging from a few days to several months. The negative consequences for ocular health are now well known and this practice has been hopefully abandoned.11 In response to these problems, a whole new generation of polymers has been developed, namely silicone hydrogels.
Silicone is highly permeable to oxygen and is an important addition to contact lens materials. This is particularly true of rigid gas permeable contact lenses, where the addition of silicone to other monomers such as fluorine or acrylate (as well as urea moieties) generates a biocompatible polymer that is rigid and tough, but gas permeable.3 Unfortunately, silicone alone is inherently hydrophobic and lipophilic: it repels water and attracts lipids. These two elements are not really compatible with contact lens wear in the ocular environment of an adult. However, because of the different composition of an aphakic infant’s tear film, this type of lens can be fitted to compensate for the absence of the crystalline lens in these children.12 For any other clinical applications, it was therefore necessary to find a way to treat silicone in a way that would minimize its negative aspects while maintaining its advantage in terms of gas permeability.
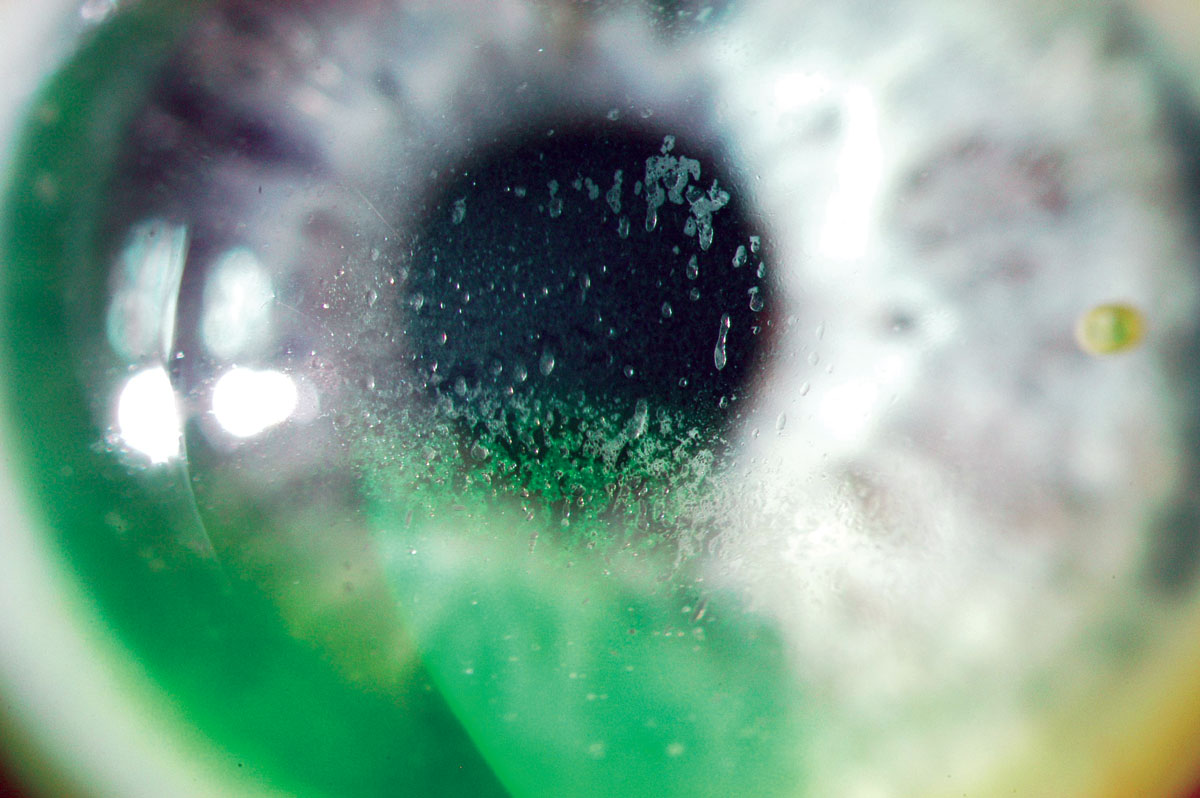 |
Fig. 3. Mucin balls trapped under the lens. Click image to enlarge. |
Kyoichi Tanaka was the first to successfully combine the TRIS monomer (its chemical name is a mouthful: tris[trimethylsiloxy] silylpropylmethacrylate) used in rigid gas permeable lenses with a hydrogel. Thus, silicone hydrogels (SiHy) lenses were born.13 A TRIS-like structure combined with polar groups made it hydrophilic.13 Another strategy created siloxy macromers containing hydrophilic polyethylene oxide (PEGS) with siloxane units. The result, at least for the first generation of silicone hydrogel lenses, was biphasic matrices with one hydrophilic and one hydrophobic phase, much like superimposed layers. The phase separation results in opacity, which was not optically suitable. The first generation of SiHy materials overcame the opacity issue by decreasing the size of the phase separation, making it smaller than the wavelength of light to ensure material clarity. Though extremely oxygen permeable, these early SiHy lenses remained relatively hydrophobic and required surface treatment, primarily using gas plasma techniques.
The introduction of silicone disrupted the established relationship between water content and oxygenation that had previously existed with hydrogels. First-generation SiHy has a very low water content, mostly because of the limited hydrophilic phases, with extreme oxygen transmissibility explodes compared to HEMA lenses (Figure 1).
Over the years, the FDA had to develop a new lens class (Group V) for SiHy materials to add to the four already present:
Group I — low-water [<50%], non-ionic
Group II — high-water [>50%], non-ionic
Group III — low-water, ionic
Group IV — high-water, ionic
 |
Fig. 4. Close-up of lipids and proteins adsorbed on a SiHy lens surface. Click image to enlarge. |
SiHy Growing Pains
Early generations of SiHy lenses (lotrafilcon A, balafilcon A, asmofilcon A) with high oxygen transmissibility were associated with a much higher modulus (and hence stiffness) than HEMA lenses, resulting in greater wearer irritation, particularly in the superior cornea or upper palpebral conjunctiva (Figure 2).14 This high modulus, coupled with spherical posterior curvature, also created new, previously unknown debris under the lens: mucin balls, which are harmless but do temporarily indent the cornea (Figure 3).15 Another new phenomenon was the accumulation of lipids (Figure 4) rather than proteins on the surface of the lens due to the attraction of silicone, as care products were not formulated accordingly.16
All of these new issues prompted manufacturers to go back to the drawing board to improve SiHy materials with the goal of making them more like HEMA hydrogels, particularly in terms of modulus and resistance to deposits. This was all the more necessary as it became increasingly clear that the new materials, in addition to improving hypoxia and hypercapnia rates, did not reduce the incidence of microbial keratitis in extended wear, while also increasing adverse events such as sterile corneal infiltrates in extended wear.17
The second generation of SiHy lenses (galafilcon A, senofilcon A) is characterized by the incorporation of a long chain of high molecular weight polyvinylpyrollidone (PVP) into the lens matrix. PVP attracts and retains water within the lens, removing the need for lens surface treatment, thereby eliminating ocular irritation and reducing lens friction on the ocular surface and conjunctiva. The presence of PVP and a greater amount of water molecules help to reduce the lens modulus (from roughly 1.3 MPa on average down to about 0.6 MPa on average). All of these modifications have had an immediate effect in improving patient comfort and reducing the mechanical problems caused by the first-generation lenses.18
It wasn’t until the third generation of SiHy lenses (comfilcon A, enfilcon A, fanfilcon A), around 2013, that the Dk and water content paradigm was broken once again. This new technology allows lenses to be made from a long siloxane macromer chain with one end modified to be hydrophilic.19 The silicone matrix becomes then naturally wettable on surface. There is no need to add a hydrophilic macromer to the silicone chains. However, it is still possible to inject other elements that promote water retention (e.g., polyethylene glycol) or, together with tear exchange, create a new, more hydrophilic environment around the lens.
In an effort to bridge the gap between SiHy and hydrogels, a new class of material, called a hypergel, has been introduced (nesofilcon A). The principle here is to develop a material that mimics the physiology of the cornea in order to optimize the biocompatibility of the lens with the ocular environment.20 The water content of the lens is thus similar to that of the cornea at 78%, close to the maximum values of hydrogel lenses, but delivering a higher gas permeability. The polymerization of the lens surface attempts to generate a barrier, thus preventing evaporation of the higher water content of the lens. Although highly hydrated, the matrix is designed to maintain a certain modulus to facilitate lens insertion and removal.
A fourth-generation material (samfilcon A) has contributed to a significant evolution in technology by introducing dual polymerization to the lens manufacturing process. While the silicone matrix body is formed after an initial polymerization, a second phase of this type not only binds but also incorporates PVP, which has the ability to attract six times its molecular weight in water, directly into the matrix. Retention time is improved, as is the surface quality of the lens.
So, it’s no longer the percentage of water in the contact lens that matters but rather the method used to maintain the most consistent hydration throughout the hours of wear. Even more recently, this technology has benefited from the injection of electrolytes, surfactants and emollients, which, when released by tear exchange and blinking, help maintain tear film homeostasis (kalafilcon A).
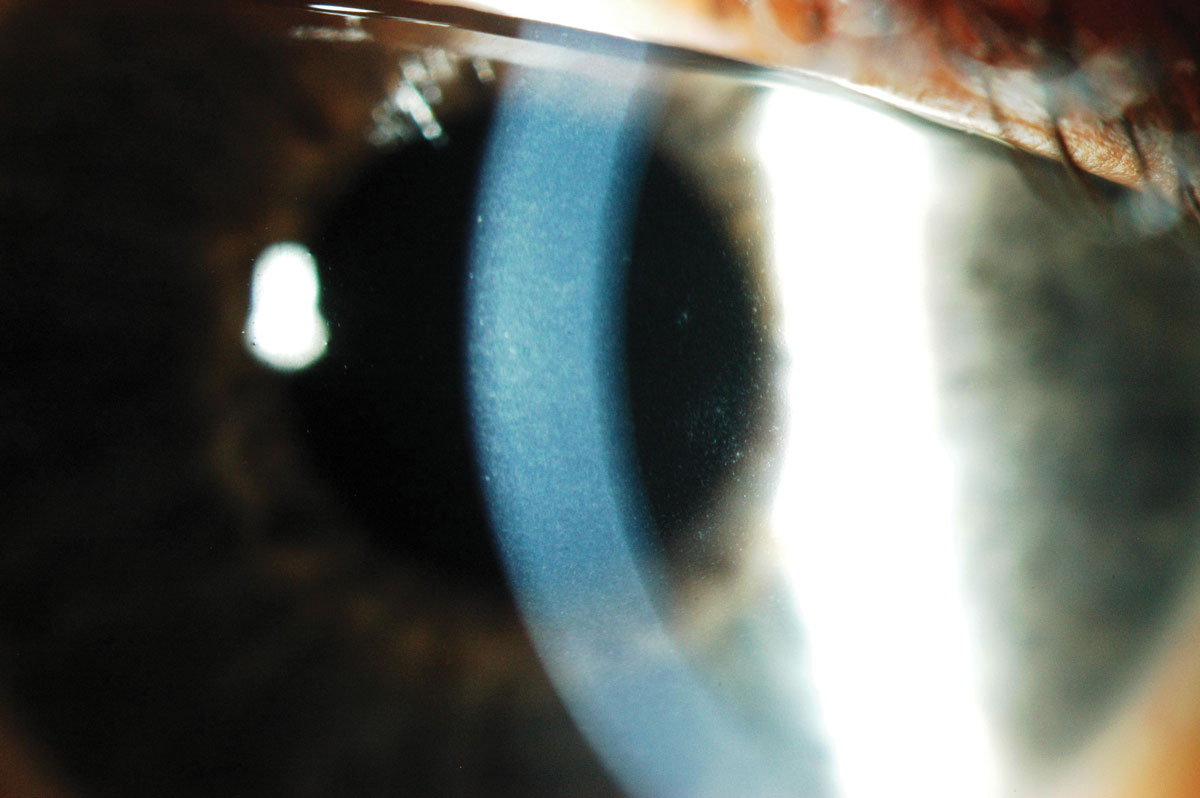 |
Fig. 5. Corneal edema and microcysts following extended wear with low Dk lenses. Click image to enlarge. |
Significant Breakthrough
Water gradient technology represents not only a new generation of lenses but also a completely new way of thinking about the relationship between the lens, the tear film and the ocular environment. With such new material, the paradigm is again broken, since there is no compromise in the relationship between oxygen and surface water, which is unique. A water gradient lens is able to achieve high oxygen permeability while maintaining a low water content at the core.21 Even better, modulus is not compromised and the lens is acting like one of hydrogel.
The core of the lens is a low water content SiHy material (33% or 55% delefilcon A or lehfilcon A). It increases toward the surface, up to 80%. At the surface, however, there is essentially no silicone at all and the content of water is almost 100%, favoring a high compatibility with the tear film. Instead, a hydrophilic gel has been designed to mimic corneal water content. The material coefficient of friction is reduced, lowering the potential adverse effects on ocular health. In the case of lehfilcon A, this surface is also designed to mimic the corneal surface architecture, which may help to resist protein and lipid deposition, considering that this lens is worn on a monthly disposable modality.
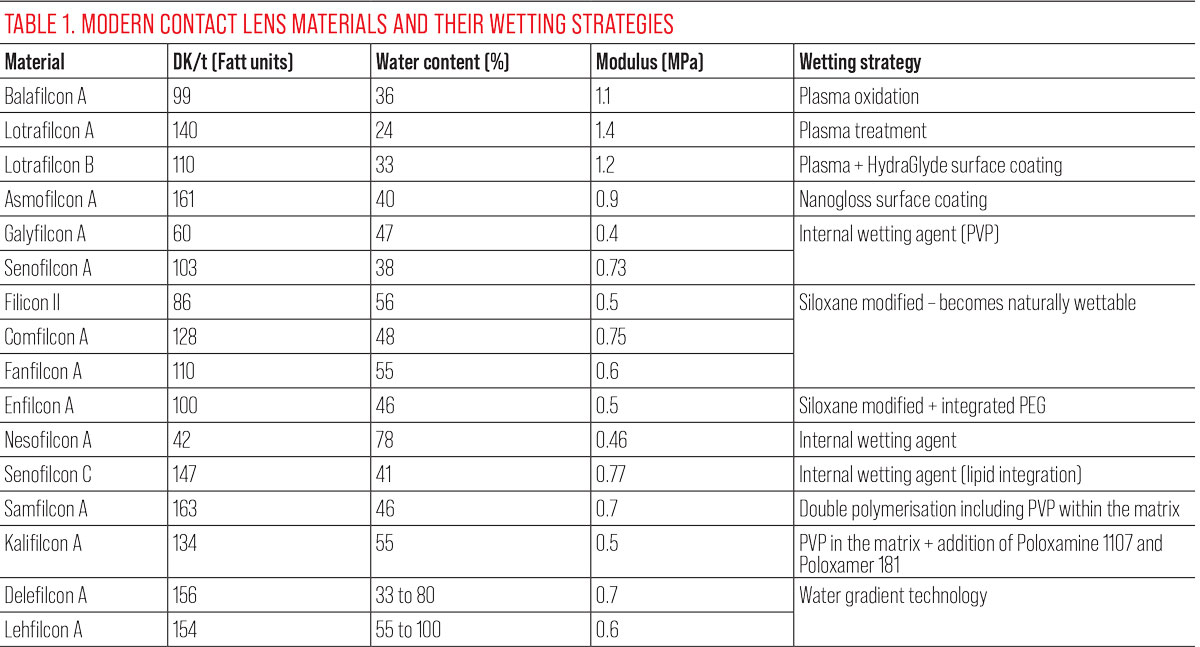
See Table 1 for a summary of lens parameters and wetting strategies.
 |
Clinical Impact: What Makes a Difference?
It’s worth remembering that the original purpose of hydrating rigid lens materials was twofold: to improve patient comfort and to maintain normal ocular health, particularly by promoting gas exchange.
When looking at HEMA lenses, it’s clear that the water content of the product has a major impact on both factors. Practitioners made their choices based on the patient’s needs and, most importantly, ocular parameters. If a patient requires a highly convex lens to correct aphakia, or a highly concave lens with thick edges, they would be fitted with the most permeable lens, which means a HEMA lens with the highest water content.
The fragility of such lenses meant that practitioners had to strike a delicate balance between the need to handle the lenses daily at the risk of tearing the lens or resorting to extended wear, which could reduce the positive impact on patients’ ocular health (Figure 5). The fit will be loose as well to allow tear exchange, which brings another source of oxygen and nutrients to the cornea. At a time when HEMA lenses were being prescribed, it’s clear that water content was a key factor in the fitting decision.
The addition of silicone to the lens matrix removes the oxygenation factor from the equation. If we look at the lenses currently prescribed (Table 1), especially the latest generation, they all far exceed the permeability requirements for daily wear and even extended wear according to the criteria of Harvitt and Bonnano (DW = 32 Fatt units; EW = 120 Fatt units).22
SiHy lenses are available in a variety of wearing modalities, including single-use lenses, which excludes extended wear by definition. Single-use lenses also improve comfort, which improves the patient experience.23 This modality limits the amount of accumulated deposits or biofilm and avoids chronic exposure to chemical solutions used to disinfect and store the lenses. The preservatives and buffers in this type of solution can increase patient sensitivity and may generate negative reactions (Figure 6).24 What’s more, a recent study showed that lenses worn for 300 hours or more (i.e., monthly disposable or conventional lenses) release plastic microparticles that can penetrate conjunctival cells and alter their metabolism.25 This also increases the inflammatory response to lens wear. This is not the case when patients are fitted into a single-use lens modality.
While the wearing modality can influence patient comfort, there are many other factors at play, keeping in mind that contact lens discomfort is multifactorial in nature.26 The water content of the lens may play a role here, especially if we consider the modulus of the lens.
Looking at the history of SiHy lens development over the past 30 years, it is clear that the composition of SiHy lenses in use today is very different from those first introduced in the early ’90s. The early designs broke the paradigm of water content vs. oxygen permeability. Technological advances of the modification of the silicone chains themselves and the use of different types of silicone have allowed for greater attraction and retention of water molecules in the lens matrix. Thus, without significantly altering oxygen transmissibility, these new structures have tended to adopt the modulus of hydrogel lenses (i.e., greater flexibility for improved comfort while maintaining a degree of rigidity necessary for easy lens handling). Consequently, today’s practitioners should be guided more by lens modulus than water content, understanding that, under the new paradigm, both are not so correlated.
Another lesson to be learned from the first years of exposure to silicone hydrogels is the importance of the mechanical aspect of their presence on ocular tissues. The first generations of lenses were characterized by a much different tribological behavior (i.e., the friction, lubrication and wear forces of two surfaces in contact) than hydrogels, notably due to the surface treatments required by the combination of silicone and hydrophilic phases. As mentioned, this mechanical stress led to episodes of giant papillary conjunctivitis, inflammation of the tarsus and conjunctiva, and even keratitis.
The latest generations of lenses offer a very different picture, and the coefficient of friction of SiHy lenses is now improved, notably if the lenses are single-use (vs. frequently replaced) and when PVP is kept in the matrix.27 In this respect, the development of water gradient technology opens up a whole new perspective, further reducing the mechanical impact of contact lens wear on the eye. Even in single-use lens modality, materials offering this technology stand out from the rest.28
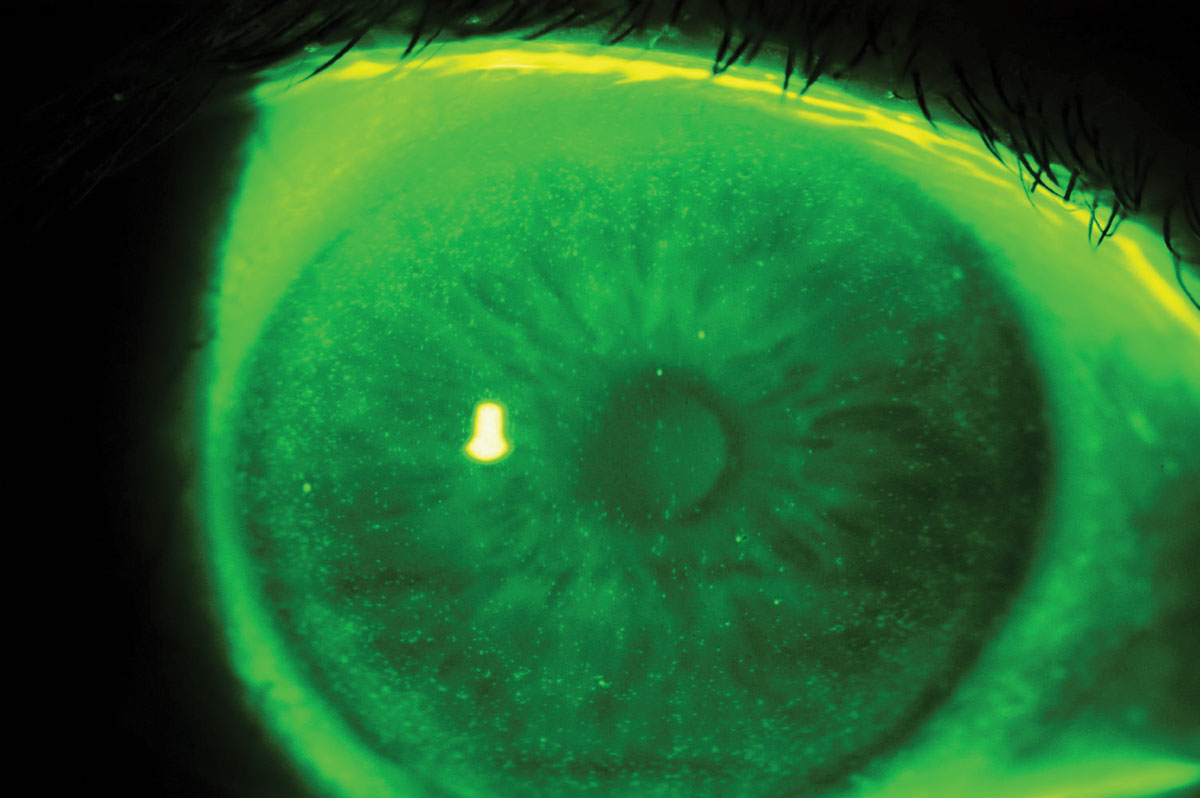 |
Fig. 6. Epithelial cells hyperfluorescence related to preservative agents reaction. Click image to enlarge. |
Takeaways
Following in Otto Wichterle’s footsteps to improve the comfort and physiological effects of contact lenses, practitioners have all the tools at their disposal to achieve these goals. Specifying the latest generation of silicone hydrogel lenses ensures safe gas exchange, while the use of lower modulus and parameters that reduce the troublesome lens/eye surface interactions associated with contact lens wear, particularly by limiting the coefficient of friction, is becoming a must. Disposable lenses that eliminate the need for solutions, and in particular those that are able to maintain a high PVP level throughout the wear period or are based on water gradient technology, seem to be the best answer we can offer to wearers looking for comfortable all-day lenses. The water content of these lenses then becomes a contributing factor, but not the only criterion to consider.
1. Caroline P, Norman C. History of contact lenses, the first 40 years of manufacturing 1887-1927. CL Spectrum. 2022;37:52. 2. Grant N, Fujimoto M, Caroline P, Norman C. History of contact lenses: a timeline celebrating the 50th anniversary of soft contact lenses. CL Spectrum. 2021;36:52-4. 3. Musgrave CSA, Fang F. Contact lens materials: a materials science perspective. Materials (Basel). 2019;12(2):261. 4. Tranoudis I, Efron N. Water properties of soft contact lens materials. Cont Lens Anterior Eye. 2004;27(4):193-208. 5. Pedley DG, Tighe BJ. Water binding properties of hydrogel polymers for reverse osmosis and related applications. British Polymer Journal. 1979;11:130-6. 6. Hatakeyema T, Yamauchi A, Hatakeyema H. Studies on bound water in poly(vinyl alcohol). Hydrogel by DSC and FT-NMR. European Polymer Journal. 1984;20(1):61-4. 7. Mirejovsky D, Patel AS, Rodriguez DD. Effect of proteins on water and transport properties of various hydrogel contact lens materials. Curr Eye Res. 1991;10(3):187-96. 8. Pritchard N, Fonn D. Dehydration, lens movement and dryness ratings of hydrogel contact lenses. Ophthalmic and Physiol Opt. 1995;15(4):281-6. 9. Rivera RK, Polse KA. Effects of hypoxia and hypercapnia on contact lens-induced corneal acidosis. Optom Vis Sci. 1996;73(3):178-83. 10. Keay L, Jalbert I, Sweeney DF, Holden BA. Microcysts: clinical significance and differential diagnosis. Optometry. 2001;72(7):452-60. 11. Holden BA, Sweeney DF, Vannas A, et al. Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci. 1985;26(11):1489-501. 12. Russell B, Ward MA, Lynn M, et al. The infant aphakia treatment study contact lens experience: one-year outcomes. Eye Cont Lens. 2012;38(4):234-9. 13. Chou B. The evolution of silicone hydrogels. CL Spectrum. www.clspectrum.com/issues/2008/june-2008/the-evolution-of-silicone-hydrogel-lenses. June 1, 2008. Accessed July 25, 2023. 14. Lin M, Yeh T. Mechanical complications induced by silicone hydrogel contact lenses. Eye Cont Lens. 2012;39(1):115-24. 15. Dumbleton K, Jones L, Chalmers R, et al. Clinical characterization of spherical post-lens debris associated with lotrafilcon high-Dk silicone lenses. CLAO J. 2000;26(4):186-92. 16. Jones L, Senchyna M, Glasier MA, et al. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Con-tact Lens. 2003;29(1 Suppl):S75-9; discussion S83-4, S192-4. 17. Szczotka-Flynn L, Lass JH, Sethi A, et al, et al. Risk fac- tors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51(11):5421-30. 18. Young G. Exploring the relationship between materials and ocular health and comfort. CL Spectrum. 2007. 19. Lira M, Lourenço C, Silva M, Botelho G. Physicochemical stability of contact lenses materials for biomedical applications. J Optom. 2020;13(2):120-7 20. Lorente-Velázquez A, García-Montero M, Gómez-Sanz FJ, et al. Comparison of the impact of nesofilcon A hydrogel contact lens on the ocular surface and the comfort of presbyopic and non-presbyopic wearers. Int J Ophthalmol. 2019;12(4):640-6. 21. Epstein AB, Karpecki PM, Pruitt J, et al. Understanding the benefits and value of water gradient contact lenses: why a water gradient daily disposable contact lens may be the best option for your patients. Rev Optom. 2014 2014/04/15/. 22. Harvitt DM, Bonanno JA. Re-evaluation of the oxygen diffusion model for predicting minimum contact lens Dk/t values needed to avoid corneal anoxia. Optom Vis Sci. 1999;76(10):712-9. 23. Fahmy M, Long B, Giles T, Wang CH. Comfort-enhanced daily disposable contact lens reduces symptoms among weekly/monthly wear patients. Eye Contact Lens. 2010;36(4):215-9. 24. Epstein SP, Ahdoot M, Koonapareddy C, et al. Toxicity of ocular preservatives and buffering agents on the ocular surface (corneal and conjunctival epithelium). Invest Ophthalmol Vis Sci. 2006;47(13):4961. 25. Liu Y, Ling X, Jiang R, et al. High-content screening discovers microplastics released by contact lenses under sunlight. Environ SciTechnol. 2023;57(23):8506-13. 26. Jones L, Brennan NA, González-Méijome J, et al. The TFOS International Workshop on contact lens discomfort: report of the contact lens materials, design and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):Tfos37-70. 27. Sterner O, Aeschlimann R, Zürcher S, et al. Friction measure-ments on contact lenses in a physiologically relevant environment: effect of testing conditions on friction. Invest Ophthalmol Vis Sci. 2016;57(13):5383-92. 28. Michaud L, Forcier P. Comparing two different daily disposable lenses for improving discomfort related to contact lens wear. Cont Lens Anterior Eye. 2016;39(3):203-9. |

