 |
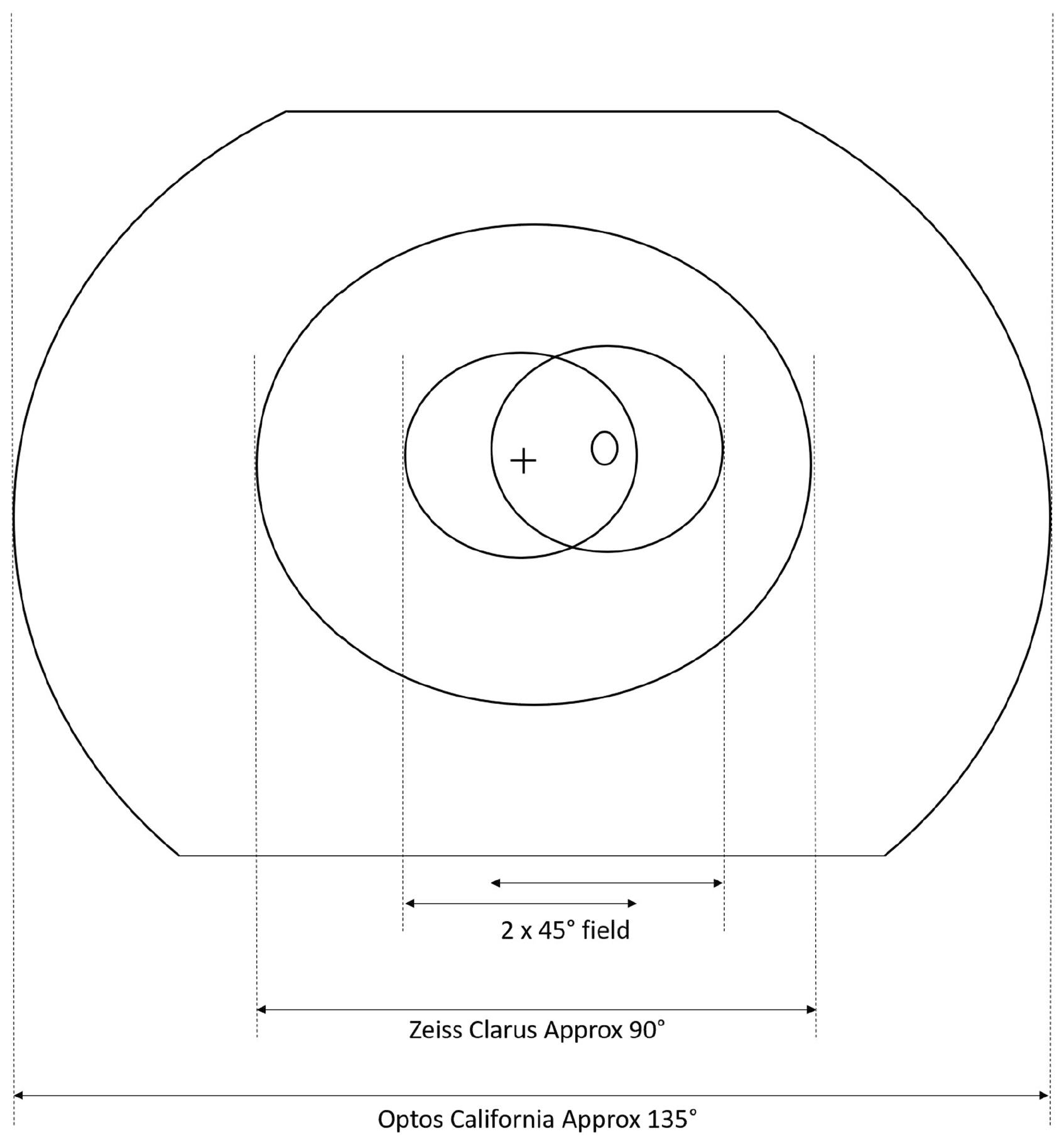
Though they don’t achieve the field of view and stereopsis of a dilated exam, both ultra-widefield imaging cameras evaluated in this study improved upon conventional nonmydriatic imaging that uses either one or two 45° fields in a screening program. Photo: Scanlon PH, et al. Eye (Lond.) Oct 8, 2024. Click image to enlarge. |
Patients tend to prefer non-mydriatic retinal assessment using a widefield imaging camera over a dilated exam, but clinicians worry that important peripheral retinal structures may be missed. Full dilated exams would be impractical for a widespread, community-based screening effort, and yet, early screening for diabetic eye disease plays a pivotal role in getting patients treated earlier in the disease course. A new study conducted in the UK sheds light on the viability and accuracy of ultra-widefield non-myd cameras when used in a screening program, finding generally good performance, especially when compared to conventional nonmydriatic imaging.
Currently, England’s NHS Diabetic Eye Screening Programme uses two-field 45° digital imaging and Scotland uses staged mydriasis with one-field 45° non-mydriatic imaging, dilating only those whose non-myd images are unable to be assessed. To improve screening endeavors, the CONCORDIA study in the United Kingdom evaluates the performance of fundus cameras and their cost-effectiveness. The first paper, published recently in Eye, assessed the DR screening performance of the Zeiss Clarus 700 and the Optos California cameras and reported that these two devices may detect more referable retinopathy.
A total of 1,497 individuals (2,993 eyes) underwent non-mydriatic photography from both cameras. Researchers compared their imaging to the reference standard: two-field 45° mydriatic digital photography. Sensitivity for any retinopathy was 94.2% for the Clarus and 91.9% for the Optos. Specificities were 87.3% and 78.1%, respectively. For referable DR, the sensitivities for Clarus and Optos were 86% and 77.6%; the specificities were 92.8% and 95.4%, respectively.
The researchers praised the high sensitivities for referrable retinopathy but expressed some reservation upon seeing the reduced sensitivities. However, a notable positive is that each camera identified additional diabetic retinopathy lesions not found by the conventional two-field 45° imaging, at rates of 4.3% for Clarus and 11.5% for Optos. Furthermore, “only 1.7% of eyes captured with the Clarus and 3.4% of eyes captured with the Optos required dilation,” the authors noted in their paper.
“The color differences did not affect red lesions (e.g., microaneurysms, hemorrhages, IRMA and new vessels) but made the interpretation of drusen and hard exudate more difficult for graders, which would have training implications” for use of such technology in a screening regimen.
The Clarus and Optos aren’t currently approved for screening use in the UK program due to concerns about central resolution. In their Eye paper, the researchers say that these initial study results are “promising for both cameras, especially when one considers how well they both performed using staged mydriasis.” The researchers noted, however, that almost 93% of participants were white, so these cameras need to be tested in other ethnic groups.
| Click here for journal source. |
Scanlon PH, Gruszka-Goh M, Javed U, et al. The Scanning CONfoCal Ophthalmoscopy foR DIAbetic eye screening (CONCORDIA) study paper 1. Eye (Lond.) 2024. [Epub October 8, 2024]. |

