The Origins and Management of Contact Lens Discomfort
Understanding how this irritating nuisance develops is the first step toward fighting its deleterious effects.
By
Release Date: November 2017
Expiration Date: November 15, 2020
Goal Statement: Contact lens discomfort can develop because of a number of issues, including poor lens wettability, low oxygen permeability or a host of environmental factors. Optometrists should be skilled at delineating these causes and the available contact lens materials so they can best target treatment and fit patients with the most approriate option.
Faculty/Editorial Board: Dan Fuller, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 55371-CL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Riviello and Michael Iannucci all have no relationships to disclose.
Contact lens discomfort (CLD) is a distinctly different entity than dry eye disease (DED), whose prevalence is estimated at 30%.1-4 Numerous studies identify CLD (prevalence 30%) as a major contributing factor to contact lens dropout rates with best estimates placing it between 12% and 51%.2,5-8 The broad range of values is a function of varying definitions of what constitutes a dropout (e.g., reduced wearing time, temporary or permanent discontinuance).
CLD creates significant burdens on patients and dramatically affects industry profitability. Identifying these patients and the factors contributing the condition will inform management decisions.
The TFOS Workshop classifies CLD by contributory factors into two large baskets: contact lens-related and environmental etiologies (each with four individual subcategories).1 This article focuses on soft lenses and reviews what we believe we know about contact lens-related factors, the contribution from environmental factors second and offers a rational, evidence-based management approach.
| Fig. 1. Example of corneal staining representative of desiccation while wearing a Group IV lens in patient with severe ocular surface disease. |
The Material World
Within the contact lens-related category of CLD are four subcategories:
(1) Surface and bulk material differences.
(2) Design differences.
(3) Fit and wear differences.
(4) Lens care factors.
Materials vary widely in both surface and bulk properties. Designs vary from rigid to hydrogel to silicone hydrogel and hybrids. Rigid gas permeable lenses represented 7% of all fits worldwide in 2016 while soft lens fits/refits constituted 91% of all fits (55% of which were silicone hydrogels).9 Substantial differences in modulus, wettability and oxygen permeability prevent direct comfort comparisons between silicone hydrogels and data from hydrogel studies.10,11 Lens attributes commonly considered potential influences on comfort include polymer composition, lubricity, water content and wettability.12-20
Various modifications to early hydrogel polymers increased their water content and hydrophilic nature. The objective was to improve wettability and oxygen permeability in an attempt to improve comfort.21 Several studies demonstrate increasing water content can lead to increased dehydration, corneal desiccation and decreased end-of-day comfort by as much as threefold in FDA Group II and IV lenses.22-25 However, these studies failed to consider the contributions from differences in lens design, leading to the conclusion on-eye bulk dehydration of materials is likely neither associated or causative of discomfort.21,25 The higher the water content, the more moisture is essentially wicked away from the ocular surface to replace moisture lost from the lens polymer through dehydration, resulting in corneal desiccation and corneal staining (Figure 1).26 Silicone hydrogel lenses tend to have water contents lower than 50%, and are classified as “low” water content by the FDA, though at least four silicone hydrogel lenses have water contents above this threshold.27
Increasing water content generally increases water and sodium chloride permeability more for ionic than non-ionic soft lenses.28 This process is an order of magnitude less for silicone hydrogels than hydrogels since it appears to be more restricted by channels in the polymer.28 The impact of ionicity on comfort has not been demonstrated conclusively.21
Research shows oxygen permeability increases with water content for hydrogel lenses, but the reverse is true for silicone hydrogel, owing to these fundamental differences.21,29,30
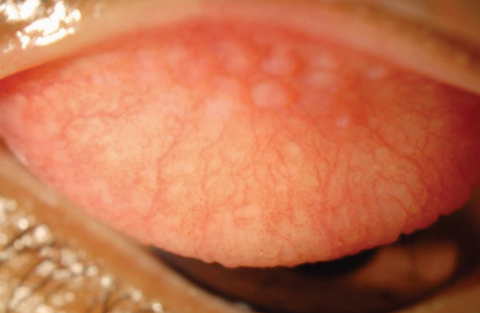 |
| Fig. 2. Contact lens-related papillary conjunctivitis while wearing silicone hydrogel. Click photo to enlarge. Photo: Christina Newman, OD |
Not long after the introduction of modern silicone hydrogels late in the 1990s, interest in the contribution of modulus or stiffness to CLD began. “Stiffness” is a function of more than the material properties such as modulus, including water content, relative thickness and geometry of the lens.12,31-33 The first generation silicone hydrogel lenses were high-modulus, low-water content designs with plasma coatings and contributed to higher rates of contact lens papillary conjunctivitis, superior arcuate epithelial lesions (SEAL) and corneal erosions and mucin balls (Figures 2 and 3).12,31,33-38 Second and third generation designs reduced the incidence of these adverse events by eliminating plasma coatings in favor of internal wetting agents such as polyvinylpyrolidone (PVP) and by increasing water contents by altering the constituent polymers, resulting in more mechanical flexibility.12 Notwithstanding some of the more prevalent adverse events associated with earlier high-modulus, low-water content designs, the Workshop on CLD concluded little difference in comfort between hydrogels and silicone hydrogels and when differences have been found, it is highly likely it resulted from methodological flaws in the study.21,39
The Workshop on CLD considered surface properties of contact lenses including friction, lubricity and surface wear (collectively referred to as tribology) and wettability.21 Studies relating these surface properties to comfort are similarly plagued by confounding lens characteristics (e.g., sag and edge profile) or methodological challenges in attempting to model on-eye performance.21,40 Notwithstanding, manufacturers have attempted to decrease friction and increase wettability at the lens surface by incorporating agents commonly used in over-the-counter wetting agents such as polyvinyl alcohol (PVA) and hyaluronic acid (HA) into their polymers.41-43 Some of these are slowly eluted or activated while blinking and over the wear cycle. More recent designs such as deleficon A and nesofilcon A possess unique surface properties which have either not shown, or not tested, comfort differences over other lenses.44,45 Interest in lid-wiper epitheliopathy and lid parallel conjunctival folds is growing as a possible predictor of CLD and may be related to studies investigating the role of frictional forces.40,46-51
 |
| Fig. 3. Corneal erosions in high modulus silicone hydrogel in superior epithelial arcuate region. Click photo to enlarge. |
Lens Design and Fit
Consideration of comfort must also include a review of fitting characteristics and lens design. Fitting characteristics are familiar to all clinicians and include coverage, movement and centration. Design attributes include edge profile, sphere, toric and multifocal parameters. Studies demonstrate a larger lens diameter can be a significant predictor of comfort.52 Most spherical soft lenses typically fall within a range of 13.8mm to 14.2mm.21 These values are above the largest diameter of 13.5mm used to study, which may explain why diameter (corneal coverage by extension) has not been shown to be associated with comfort in more recent studies.40,52-55
Lens movement contributes to tear exchange but the contribution of lens movement (or lack thereof) to comfort is somewhat murky. Two large retrospective studies demonstrate “loose” fitting lenses are associated with discomfort and “tighter” lenses are not, but no clear consensus exists on the minimum difference between base curves which elicit awareness.21,40,55,56
Centration has not been studied in relation to comfort independent of looseness/tightness of fit and researchers suggest small amounts of decentration (<0.3mm) are unlikely to affect comfort.21,40 A well-centered lens, with coverage and minimal amounts of movement (about 0.5mm to 1.0mm) in primary gaze, is associated with better comfort.40,55
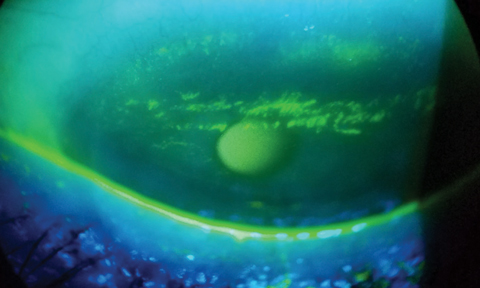
Additionally, edge profiles appear to matter, with thin knife edges consistently demonstrating better comfort than chisel or rounded edges even though they have a higher association with paralimbal conjunctival staining (Figure 4).57-59 Chisel edge profiles are associated with higher frequencies of conjunctival indentation, which is associated with discomfort (Figure 5).60
Regarding lens designs, prism ballasted toric lenses may be more likely to elicit symptoms of discomfort confused with dryness and may represent lid-lens interactions, but direct comparisons with spherical lenses are rare to absent.21,61-63 Multifocal contact lens comparisons with spherical lenses are similarly rare but have shown no difference in comfort.64
  |
| Fig. 4. Example of difference in edge profiles on -3.00D sphrerical lens designs, demonstrating a rounded, thicker edge in comifilcon A (left) and knife edge in senofilcon A (right). Click photos to enlarge. |
Modality and Wear Schedules
Research does not show that daily wear is any more comfortable than extended wear (except upon waking), but that may be due to a shortage of robust clinical studies.21 Though duration of lens wearing experience has a role in adaptation, it is difficult to separate the impact of duration from frequency of replacement or age.21 Multiple circumstantial studies demonstrate improved comfort with increasing frequency of lens replacement, but it is difficult to separate out the findings from confounding variables of differences in lens material or care systems in comparison studies.21 Masked, randomized, controlled studies are lacking. End-of-day comfort is clearly differentially worse for all contact lens wearers compared with non-wearers, with increasing symptoms in both groups.65–67
Lens Care
It may seem counterintuitive, but regardless of the nature of deposits, they have not directly been implicated in decreasing comfort.21 However, the subject of lens care solution interactions and their impact (positive or negative) on comfort is a subject of ongoing debates. It remains very difficult to assess individual impacts of constituent agents due to the confounding influences of their interactions, compliance methods and conflicts between in vitro and in vivo performance differences. Comparison studies exist, but are limited by the number of possible lens-solution combinations tested. Biocides include hydrogen peroxide-based, polyhexamethylene biguanide PHMB-based, Polyquad-based, and dual disinfection systems. The reality is that all FDA-approved systems have met current standards for biocidal efficacy against the challenge panel of organisms.
Peroxide-based systems begin at 3% concentration (30,000 ppm) and must be neutralized to 100 ppm, though threshold sensitivity is between 50 to 300 ppm or discomfort will be reported.21 As previously mentioned, there are few direct comparison studies that control for potential confounding variables. Nonetheless, there is a “suggestion” peroxide-based systems provide better comfort with the limited data available.68,69 Comparisons of comfort using PHMB- and Polyquad-based systems have occasionally favored Polyquad- based systems but the majority view is there is no difference in comfort.70-75 Preservative uptake/release and induction of corneal staining with possible CLD is unique to each lens-solution combination, making it relatively unpredictable. Modern multipurpose solutions incorporate surfactants as both detergents and wetting agents. There is compelling evidence these can contribute to patient comfort by increasing the hydrophilicity of the lens surface, particularly in silicone hydrogels.21 Similar findings exist for the surfactants and wetting agents commonly added to blister pack solutions.
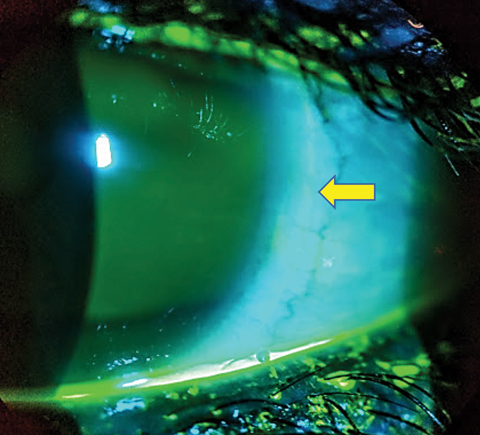 |
| Fig. 5. Partial arcuate indentation of the nasal bulbar conjunctiva related to edge profile (yellow arrow). Click photo to enlarge. |
Other Factors
We have summarized broadly the evidence supporting contact lens-related factors that may contribute to CLD. It may be unsatisfying, but nonetheless true, that there is no one factor most responsible for CLD. Rather, multiple lens and solution factors contribute to CLD. Factors such as discussion of wearing times, replacement intervals and modality must include consideration of environmental factors, controllable or uncontrollable. This section will summarize some of the more salient take-aways.
Non-modifiable patient factors surveyed in the Workshop on CLD included sex, age, ethnicity, tear film, blink characteristics, comorbidities and allergies.2 The authors found females may have higher rates of CLD, but this does not appear to predicative of dropout. Younger patients report CLD more often than presbyopia patients do, particularly in hydrogels. Assessments of tear film volume and stability including phenol thread test, tear meniscus, noninvasive TBUT, and pattern of breakup correlate with CLD. Little evidence exists supporting an association between changes in blink rate and CLD, but stability of the tear film in the interblink interval may be related to CLD. Seasonal allergies may be associated with CLD. Ethnicity appears not to be associated with CLD, with little evidence supporting a relationship between systemic disease and CLD.2
Modifiable patient factors included medications, dietary habits, smoking, cosmetics, compliance and psychological factors. Use of oral contraceptives and isotretinoin have been associated with CLD but no other agents are conclusively documented as contributing to CLD. Poor compliance with replacement intervals is associated with CLD. There is little evidence to support the notion that dietary intake and fluids influence CLD. The influence of smoking on CLD lacks evidence. Little evidence supports an association between cosmetic use and CLD. Psychological factors have not been found to be associated with CLD.2
| Table 1. Management Strategies Based on 2013 TFOS Workshop on Contact Lens Discomfort76 | |
| Treatment strategy | Specific intervention |
| Replacement frequency | Increase |
| Material | No clear rule; switch to from higher to lower modulus within silicone hydrogel; switch from silicone hydrogel to hydrogel (or reverse); consider lower water content hydrogels |
| Add internal wetting agents | Consider silicone hydrogels lenses with PVP, PVA, hyaluronic acid. |
| Add external wetting agents | Preservative-free rewetting drops; modern MPS and peroxide disinfection systems offer surfactants and wetting agents |
| Elimination of the care system | Daily disposables |
| Change lens parameters | Steeper base curves; aspheric base curves; larger diameter; knife edge |
| Nutritional supplementation | Omega-6 in evening primrose oil |
| Punctal occlusion | Occlude upper and lower |
| Topical agents | Cyclosporin-A; lifitegrast |
| Digital device use and occupational exposure | Avoidance |
| Consider changing from GP to soft or vice versa | Consider in relationship to needs |
| Reduce wearing time | Adjust to find optimum level |
| Other | Orthokeratology; refractive surgery; spectacles |
Ocular enviromental factors. Contact lens wear alters multiple aspects of the ocular anatomy and physiology, including: thinning and destabilization of the tear film; increasing tear osmolarity; loss or shortening of the meibomian glands; alterations to corneal sensitivity; cellular changes in the corneal and conjunctival epithelium.2 Among these, the presence of lid-parallel conjunctival folds, conjunctival metaplasia, decreased goblet cell density, meibomian gland dysfunction and lid-wiper epitheliopathy have been shown to be associated with CLD.2
External environmental factors reviewed included relative humidity, temperature, climate, air quality, atmospheric pressure and occupation. Among these, reductions in relative humidity, increased air movement and activities which reduce blink rates such as digital device use may all contribute to CLD.2 The remaining factors lack evidence or are equivocal.
Management of CLD
History-taking continues to be the foundation of all patient encounters. Certain risk factors help identify wearers at risk for CLD. Younger patients are at increased risk; end-of-day discomfort or discomfort upon insertion; specifics on lens parameters; wearing time; replacement interval; care system; use of adjunctive wetting agents; compliance; occupation and vision demands; coexisting disease; allergies; and current medications.76
Strategically manage all underlying non-lens factors contributing to CLD, including diseases that contribute to ocular surface disease. Identify instances of inappropriate medication use, overuse or abuse, which may destabilize the tear film. Treat coexisting lid, tear film, cornea or conjunctival disease. Manage lens-related issues, including condition, fit and interactions with the eye. This approach is basic to all contact lens examinations.76
When confronted with lens wearers symptomatic for discomfort, one or more of the recommendations from the Workshop on CLD cited in Table 1 may be employed based on your clinical assessment of the patient.76 Not all strategies are supported by “level I” evidence and a combination approach is often necessary. Implementing too many strategies at once and failing to manage underlying conditions may confuse your management plan. Be judicious and methodical.
CLD is ubiquitous, but we have never had more sophisticated lens and solution options. As our understanding continues to grow so, will our ability to provide wearers with lifelong comfortable, clear vision. Identify patients at risk and those with contributory conditions. Apply evidence-based management strategies in a methodical manner, increasing the probability for long-term success.
Dr. Fuller is chief of Cornea & Contact Lens Service and founding supervisor of the Cornea & Contact Lens–Refractive Surgery residency at Southern College of Optometry.
1. Nichols J, Willcox M, Bron A, et al. The TFOS international workshop on contact lens discomfort: executive summary. Investig Opthalmology Vis Sci. 2013;54:TFOS7. |
