Although a connection between eye firmness and blindness was recorded as far back as the 1600s, we still have no perfect method to measure intraocular pressure (IOP); all current methods are influenced by various ocular and non-ocular factors and can only give us an estimate of the intraocular pressure.
Accurate and precise IOP readings are imperative to evaluate a patient’s risk of progressive optic nerve damage. Inaccurate or inconsistent IOP measurements prevent the clinician from making accurate treatment and management decisions and may put the patient at risk for visual field loss. Clinicians need to develop a consistent, reproducible and reliable technique for obtaining IOP measurements so that they can be compared with confidence over time.
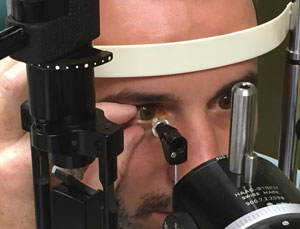 | |
| When holding the patient’s lids open, don’t inadvertently apply pressure to the globe. |
Here are some helpful recommendations to keep in mind when measuring a patient’s IOP (unless otherwise noted, recommendations relate to Goldmann applanation tonometry, GAT):
DO consider the patient’s position, comfort and clothing prior to checking IOP.
Make sure to position the patient correctly in the slit lamp without discomfort. The head and chin should be in contact with the forehead and chin rests and the lateral canthi should be aligned with the line on the slit lamp’s frame.
Encourage the patient to breathe normally. It’s common for patients to hold their breath out of anxiety, poor positioning, or both, during an IOP measurement. This may increase venous pressure from the Valsalva maneuver, resulting in an inaccurately high reading.1
Lastly, studies have found tight collars, ties or other restrictive clothing around the neck may cause an increase in venous pressure when the patient extends his or her neck forward, resulting in an inaccurately high IOP reading.2 Have the patient loosen any collars or neckties that may be too restrictive to obtain an accurate measurement.
DON’T apply pressure to the globe. DON’T let patients squeeze their eyes shut.
External pressure to the globe can influence the measurement. This can occur from the patient squeezing his or her eyes or the examiner inadvertently applying pressure to the globe.
Many patients need assistance adequately opening their eye during tonometry. Small fissure sizes, dermatochalasis, long eyelashes, blepharospasm and the reflex or fear of an object close to the eye can all make obtaining a pressure reading difficult and artificially high.
Common Types of Tonometry • Goldmann applanation tonometry (GAT), introduced in the 1950s, is currently regarded as the reference standard. The method involves contacting an anesthetized cornea with a tonometer tip approximately 3.06mm in diameter and using fluorescein dye in the precorneal tear film to determine the force necessary to flatten the cornea. The size of the tonometer tip is deliberate to minimize the impact of the corneal resistance and the surface tension of the tear film.7 Two semicircles are visible through the bi-prism. The examiner turns the tension knob that alters the force applied to the cornea, and the IOP is determined in mm HG when the internal aspect of the two semicircles are in contact with each other. • A Perkins tonometer resembles a GAT and uses the same applanating prism, but is portable and can be used on patients who are not being tested with a slit lamp in the office, those with physical limitations preventing them from positioning in a slit lamp and those being tested in the supine position. • Non-contact tonometry (NCT), also known as the “air puff test,” uses increasing air intensities to flatten the apex of the non-anesthetized cornea. The force used to flatten the cornea is detected by sensors, recorded and converted to mm Hg. The benefit to NCT is that no anesthetic is required since the cornea is not contacted during the procedure. • The Tono-Pen (Reichert) is a handheld electronic device that uses a small plunger to gauge the resistance of an anesthetized cornea when in contact. It has a known area of flattening and correlates well with GAT in “normal” IOP ranges. The device is easily portable and is most advantageous when used on scarred or edematous corneas. However, it uses a disposable latex tip and is contraindicated if the patient has a latex allergy. Rebound tonometry assumes that harder eyes (those with a high IOP) will induce a more rapid deceleration of a probe than a softer eye (those with a low IOP). The rebounding velocity is then converted into mm Hg. • Icare (Icare), the newest handheld device in this category, measures the induction current created when the plastic-tipped metal probe rebounds off the cornea and is driven back into the device. This method measures the IOP relatively quickly and doesn’t require anesthesia. |
Avoid applying pressure to the globe when holding the lids open, and make sure the contact area is free of eyelashes. Lift the upper lid with your index or middle finger without pinning the eyelid against the eyeball. Alternatively, use a cotton-tip swab to roll or hold the upper eyelid against the superior orbital bone. The lower lid may need to be stabilized as well with the clinician’s thumb.
Be sure to obtain the pressure reading on the central cornea with the eye in primary gaze. Instruct patients to keep both eyes open and concentrate on a distant target (such as a fixation light or a point past your ear).
Lastly, patients may find it easier to keep their eyes open if they concurrently open their mouth. This may not be practical in the slit lamp, but it can be helpful when using handheld devices like the Tono-Pen or Icare.
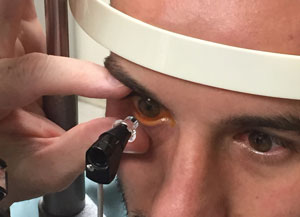 | |
| Make sure the contact area is free of eyelashes and the patient doesn’t squeeze his eyelids shut. Hold the patient’s lids open gently to avoid any pressure on the eye. |
DO use the appropriate amount of fluorescein.
During GAT and Perkins tonometry, it is important to instill the correct amount of fluorescein in the eye, using either Fluress (fluorescein sodium and benoxinate hydrochloride, Akorn) or fluorescein strips with a topical anesthetic.
Placing too much fluorescein into the eye will make the mires too thick, causing the IOP reading to be overestimated. Have the patient blink and wipe his or her eyes if too much fluorescein is present.
Instill additional fluorescein if an inadequate amount is in the tear film; otherwise, the mires will appear thin and the measurement will be underestimated.
When measuring the IOP, the light source is also important. The cobalt blue light source should be bright, diffuse and obliquely directed toward the tonometer tip.
DON’T use contact tonometry on those with active corneal disease.
Use non-contact methods, like Tono-Pen, Icare or NCT, on patients with active corneal infections or corneal epithelial defects. Also, take care with patients who have recently suffered from an ocular chemical burn or have a history of recurrent epithelial erosions.
While IOP measurements are important, you must consider whether it’s necessary in the balance of causing more corneal insult, delaying healing or increasing a patient’s chances of corneal infection. It is important the instrument of choice is clean and disinfected.
Take note of the location and size of the corneal disease and choose the best method. Remember, if the Tono-Pen device is used on a non-central corneal location, the IOP reading will likely measure higher due to differences in corneal properties of the peripheral cornea.
Tonosafe disposable prisms should be considered when using GAT and Perkins tonometers. Tonosafe disposable prisms reduce the risk of spreading infection to the other eye or another patient. While some clinicians use the disposable prisms on every patient, these prisms are especially good to use on patients with non-central corneal disease or an ocular infection that does not involve the cornea. These disposable prisms can also eliminate the need to disinfect the tonometer prism between each patient and decreases the risk of spreading infection.
DO document and consider corneal characteristics.
It is important to evaluate the cornea prior to IOP measurement to rule out contraindications to tonometry or note findings that may influence the measurement. Corneal properties that affect resistance to applanation can influence the measurement; for example, scarring can result in an artificially high reading, while edema can cause a lower reading. Lastly, look for signs of prior refractive surgery, as the cornea will be thinner than prior to surgery, and IOP readings may be artificially low.
CCT’s Impact on IOP and Glaucoma Two recent studies explore the link between central corneal thickness (CCT) and glaucoma, underscoring the need to perform baseline CCT readings before and during glaucoma treatment.1,2 Researchers at the Kaiser Permanente Northern California health plan system looked at data from 81,082 patients and found that female sex, increased age and black race were associated with thinner corneas in those with or without glaucoma.1 The gender and age connections weren’t statistically significant; but the fact that blacks, and to some extent Hispanics, tended to have thinner corneas was notable, especially considering these groups are known to have a higher prevalence of glaucoma. In these patients, CCT thinning accounted for almost 30% of the increased risk of glaucoma compared with whites. Researchers are still unsure whether there’s a direct causal link between a thin cornea and the pathophysiological mechanisms of glaucoma—or whether these factors are simply coinherited. “I would think it’s coinherited,” says Theodore Perl, MD, medical director at Corneal Associates of New Jersey in Fairfield, NJ, and The Keratoconus Center of New Jersey. “It doesn’t cause it, and it’s not directly related. It may serve as a marker or mediator. If your cornea is thinner, then look for a possible second association, which might be susceptibility to glaucoma.” Corneal thinning has further clinical relevance for glaucoma in that the use of prostaglandin analogs (PAs) and other topical glaucoma medications have been associated with reduced CCT. A recent study from Germany sought to determine whether long-term treatment using these agents had a significant effect on CCT as measured by partial coherence interferometry.2 The researchers found that over a mean of 4.2 years, CCT decreased for those treated with PAs and combination therapies with PAs, CAIs and beta blockers. Additionally, corneal thinning has been shown to result in underestimated IOP readings—a 25μm reduction of CCT can lead to about a 1mm underestimation of pressure. Thus, the researchers warn, “follow-up intraocular pressure measurements may be underestimated for eyes treated with the aforementioned treatment regimens if central corneal thickness is not measured on a regular basis.” For the typical person with no risk factors and with normal IOP, there’s no need to be too concerned about the corneal thickness, Dr. Perl says. But the clinical picture changes for a patient with a history of, or risk for, glaucoma. “In those cases, you really want to be sure that you do the central corneal thickness measurement as part of the intraocular pressure assessment, so you can put it in perspective and say, ‘Ah, his pressure is normal today, it’s 18mm Hg; but that’s kind of borderline, and maybe that 18mm really is 22mm because his cornea is thinner.’” Dr. Perl says. “So the optometrist who’s managing these kinds of problems should at least have that in the back of their minds in terms of when it’s important to do it and what the [patient’s situation] is.” 1. Wang SY, Melles R, Lin, SC. The impact of central corneal thickness on the risk of glaucoma in a large multiethnic population. J Glaucoma 2014;23:606-612. |
Take into consideration the central corneal thickness, as this can influence common forms of tonometry. Specifically, GAT assumes the central cornea is approximately 520µm in thickness. The more the central corneal thickness measurement deviates from this assumption, the less accurate the measurement—thicker corneas will be overestimated and thinner corneas will be underestimated. Several nomograms are available to adjust the reading based on the central corneal thickness, but none have been validated and universally endorsed. Often it is useful to qualitatively consider the cornea as thin, thick or average. Understanding the general principle is most important; assigning a specific number value to adjust the IOP measurement is not.
DON’T forget to take into account corneal astigmatism.
With Goldmann applanation tonometry, corneal astigmatism greater than three diopters may influence the IOP measurement. One study found the IOP was best measured at a different angle, approximately 43° from the major axis of astigmatism (in minus cylinder), which is marked on the Goldmann tonometer prism holder with a red line.3 If the prism orientation adjustment is not made, the different curvatures of the cornea will influence the IOP reading; low IOP readings will be recorded with with-the-rule astigmatism, and higher readings will be recorded with against-the-rule astigmatism.1 Take note, the mires will appear obliquely oriented and may be more difficult to adjust because the slit lamp does not have the capability to adjust the mires diagonally.
  | |
| Don’t place too much fluorescein into the eye, which will make the mires too thick (left) and cause the IOP reading to be too high. Use the appropriate amount of fluorescein (right) to keep the mires in line. |
Instead of using GAT for patients with astigmatism, consider using the Tono-Pen, a pneumotonometer or other devices if a patient’s cornea is significantly irregular (from refractive error, scarring, ectasia, etc.).
DO make sure your instrument is calibrated.
Miscalibration of the instrument can result in systematic IOP measurement inaccuracies even with flawless technique. Check the device’s operator’s manual to learn how to calibrate it.
You should calibrate your instruments at least yearly and preferably twice per year. Tono-Pen requires more regular calibration and will remind the user that calibration is needed.
DON’T make significant treatment changes based on one IOP measurement.
Because IOP fluctuates over diurnal and nocturnal periods, you should rarely base significant changes in the management or treatment of a patient on one pressure reading alone. Repeat the IOP measurement at different times of day or on a different day to account for the normal IOP changes, which can fluctuate 2mm Hg to 6mm Hg in a 24-hour period.4,5
Factors impacting IOP fluctuations are not well understood, and artifacts can potentially contribute to the IOP measurements. Even experienced clinicians repeat a measurement if accuracy is suspect.
Multiple measurements are recommended to obtain an accurate estimate of the mean IOP in tonometry devices other than Goldmann applanation. For instance, non-contact tonometers (NCT), as compared with GAT, may underestimate high IOPs and overestimate low IOPs. Ideally, take an average of two to three readings depending on the NCT model; then if IOPs are considered high, low or not reproducible, perform GAT.
Similarly, the Tono-Pen and Icare devices provide relatively accurate measurements when IOP is within normal ranges; but when possible, use GAT to confirm high or low readings.
DO consider new methods.
Researchers and clinicians are constantly trying to develop better methods than are currently available to record intraocular pressures. Other devices to consider include:
• The Ocular Response Analyzer (Reichert) is similar to NCT, but accounts for the cornea’s viscoelastic properties, or corneal hysteresis. Mathematical equations are then used to “correct” the IOP based on the elasticity of the cornea.
• The Pascal Dynamic Contour Tonometer (Ziemer) uses a curved probe, larger than a GAT tip, to measure IOP via hydrostatic coupling; it has demonstrated exceptionally accurate measurements compared with other current methods.6 This method also takes into account corneal properties, but does take longer to perform (approximately 2.5 minutes) than other methods.6
• Twenty-four hour monitoring devices would allow clinicians to analyze IOP fluctuations throughout the day, rather than momentary “snapshots” taken during office hours. For example, the Triggerfish (Sensimed) contact lens contains sensors to monitor changes in the curvature of the cornea, which researchers presume is affected by IOP changes; however, the findings have yet to be validated.
• Surgically-implanted IOP sensors are also being studied, particularly for patients already undergoing ocular surgery. The accuracy and precision of these devices is currently under investigation.
Intraocular pressure is an important exam element when evaluating a patient, and it should be done carefully and accurately when warranted. Different methods and technologies have recently been introduced or are in development to help clinicians obtain more precise measurements.
Still, IOP should not be the only finding that determines a patient’s likelihood for progressive disease. Many other findings—family history, central corneal thickness, other corneal properties, optic nerve head appearance, visual field findings, among others—should also be considered when assessing a patient’s risk for glaucoma.
Dr. Townsend is a staff optometrist at Bascom Palmer Eye Institute, Miller School of Medicine at the University of Miami in Miami, Fla. Dr. Townsend sees patients with both the glaucoma service and comprehensive service.
Dr. McSoley is a staff optometrist at the Bascom Palmer Eye Institute.
1. Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993 Jul-Aug;38(1):1-30.2. Bain WE, Maurice DM. Physiological variations in the intraocular pressure. Trans Ophthalmol Soc UK. 1959;79:249-60.
3. Goldmann VH, Schmidt T. Uber applanationstonometrie. Ophthalmologica. 1961;141:441-56.
4. Health Quality Ontario. Diurnal Tension Curves for Assessing the Development or Progression of Glaucoma: An Evidence-Based Analysis. Ontario Health Technology Assessment Series. 2011;11(2):1-40.
5. Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960;64(4):494-501.
6. Okafor KC, Brandt JD. Measuring intraocular pressure. Curr Opin Ophthalmol. 2015 Mar;26(2):103-9.
7. Gloster J, Perkins ES. The validity of the Imbert-Fick law as applied to applanation tonometry. Exp Eye Res. 1963 Jul;2:274-83.

