Take a Clinical Approach to Anterior Uveitis
The ultimate goal is to narrow down symptoms to effective treatment.
By Dominick L. Opitz, OD
Release Date: February 15, 2019
Expiration Date: February 15, 2022
Estimated Time to Complete Activity: 2 hours
 |
Jointly provided by Postgraduate Institute for Medicine and RGVCE
Educational Objectives: After completing this activity, the participant should be better able to:
- Demonstrate knowledge of the ocular signs and symptoms, as well as systemic associations, related to anterior uveitis.
- Use common lab tests to help localize the etiology of the uveitis.
- Perform a dilated fundus examination for all patients who present with anterior uveitis.
- Define the appropriate topical or systemic anti-inflammatories and, importantly, the frequency of dosing for managing anterior uveitis.
- Describe the use of cycloplegia to reduce pain, prevent synechiae formation and re-establish the blood-aqueous barrier.
Target Audience: This activity is intended for optometrists engaged in the care of patients with uveitis.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and RGVCE. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Dominick L. Opitz, OD, associate professor, the Illinois College of Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 60776-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Opitz: Consulting fees from Valeant/Bausch + Lomb and fees for non-CME/CE services from Valeant/Bausch + Lomb.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The RGVCE planners, managers and editorial staff have nothing to disclose.
Anterior uveitis (AU) causes frustration for patients and clinicians alike. The inconvenient onset and often severe symptoms result in urgent office visits, disrupting the patient’s daily life as well as the provider’s office and clinic flow. The rigorous treatment, frequency of follow-up visits and risk for recurrence can further add to the frustration. Failure of timely diagnosis as well as inefficient and/or ineffective treatment can have serious sight-threatening complications.
Following a logical, clinical approach to AU will help the primary eye care provider effectively and efficiently manage anterior uveitis (Figure 1).
 |
| Fig. 1. Following this chart may offer an effective approach to the diagnosis and management of anterior uveitis where the ultimate goal is to narrow down symptoms to effective treatment. |
Clinical Examination
Anterior uveitis is inflammation that arises in the anterior segment, including the iris and anterior ciliary body. AU is the most common form of uveitis, accounting for 50% to 60% of all uveitis, and is the most common type of ocular inflammation optometrists will encounter.1-4
An estimated 30% to 52% of AU cases have an underlying systemic etiology, and the primary eye care provider is often the first health care provider to aid in the systemic disease.4 Effective treatment and management of uveitis should include controlling the signs and symptoms of the disease, preventing ophthalmic complications and identifying any systemic underlying etiology.
The symptoms of AU include pain, redness, reduced vision and photophobia, but these vary depending on the clinical course (acute vs. chronic) of the uveitis.5
The Standardization of Uveitis Nomenclature (SUN) Working Group developed descriptors of uveitis to clarify the onset, duration and course.6 The onset is described as either sudden or insidious, whereas duration is defined as limited (≤3 months duration) or persistent (≥3 months duration).6 These descriptors are then used to define the clinical course—acute, recurrent or chronic.
Acute uveitis occurs with sudden onset and limited duration.6 Recurrent uveitis is characterized by repeated episodes separated by periods of inactivity without treatment for three or more months.6 Chronic uveitis describes cases in which relapses occur less than three months after discontinuing treatment.6
The pathophysiology of uveitis refers to the type of inflammatory cells produced, either granulomatous or non-granulomatous.
Granulomatous inflammation is characterized by inflammatory cells of the mononuclear phagocyte system that take the form of macrophages, epithelial cells and multinuclear giant cells.7 This form of inflammation is commonly a manifestation of infectious, toxic, autoimmune or neoplastic origin.7 Granulomatous inflammations can also be a critical sign of chronic inflammation as seen with chronic systemic autoimmune conditions.
Non-granulomatous inflammation allows protein and white blood cells to enter into the aqueous humor, causing the classic signs of anterior chamber cells and flare. AU resulting from non-granulomatous inflammation is more commonly seen in non-infectious underlying etiologies.
The clinical signs associated with AU—manifested in the conjunctiva, cornea, anterior chamber and iris—are a direct result of inflammation in the anterior segment that results in a breakdown of the blood-aqueous barrier. Inflammation of the conjunctiva in anterior uveitis is often described as perilimbal injection or circumlimbal injection.5 If the inflammation is granulomatous, conjunctival nodules may develop. These nodules are collections of inflammatory material within the bulbar and/or palpebral conjunctiva.
Corneal findings associated with anterior uveitis may include corneal edema, but the most common corneal finding is keratic precipitates (KPs). These are collections of inflammatory cells that accumulate on the endothelium.8 New KPs are typically white in color with smooth borders. As KPs become more chronic, they may appear pigmented with irregular borders.
KPs can be classified as granulomatous or non-granulomatous. Granulomatous KPs are large with a waxy or “mutton fat” appearance (Figure 2).5 Their presence is highly suggestive of an underlying infectious etiology, but they can also be seen in chronic autoimmune conditions such as sarcoidosis. In contrast, non-granulomatous KPs are small, fine and white, and found more in non-infectious etiologies.
The hallmark sign of AU is the presence of anterior segment inflammatory cells.5 These are white blood cells that circulate in the aqueous humor. The SUN Working Group developed a uniform grading scheme for anterior chamber cells (Table 1).6
 |
Anterior chamber flare occurs from an influx of protein from the uveal blood vessels into the anterior chamber, which results from increased vascular permeability of the uveal vasculature in the anterior chamber.9 Flare is graded according to the SUN grading scale: no flare is grade 0; faint/barely present is grade 1+; moderate with iris and lens details clear is grade 2+; marked with iris and lens details hazy is grade 3+; intense with formed fibrin or plastic aqueous humor is grade 4+.6
When anterior chamber inflammation is severe and there is significant breakdown of the blood-aqueous barrier, extensive protein leakage occurs, which causes the development of fibrin membrane across the pupil when the flare is 4+ (Figure 3).
With AU, changes to the iris—such as miosis, iris nodule formation and iris synechia—may occur. Miosis typically occurs from spasms to the iris sphincter or from distension of iris blood vessels.10 Iris nodules are accumulations of inflammatory cells and may occur at the iris margin of the pupil or in the iris stroma. Nodules at the pupil margin are termed Koeppe nodules and are found in both granulomatous and non-granulomatous inflammation whereas Busacca nodules are found in granulomatous inflammation and are located in the iris stroma.5 Other iris findings in AU may include irido-corneal adhesions, termed peripheral anterior synechia.5,8
Posterior synechia are adhesions of the iris and anterior lens that generally develop from Koeppe nodules or a thickened iris due to inflammation. Localized diffuse or sectoral iris atrophy may also occur in AU and is more common in chronic and recurrent uveitis or when the underlying etiology is viral in nature.
Intraocular pressure (IOP) is historically described as low in anterior uveitis—but IOP may be normal, low or high depending on the underlying cause, the clinical course and/or the number of inflammatory cells affecting the outflow of aqueous humor through the trabecular meshwork (TM).5,8 It is more common for IOP to be low during an acute phase due to secretory hypotony of the ciliary body. Elevated IOP is more likely to occur in chronic AU when the TM becomes overwhelmed with inflammatory material or pigment. If the underlying etiology is due to herpetic eye disease, trabeculitis frequently occurs, which affects outflow and causes elevated IOP.
Although vitreous cells are the hallmark sign of intermediate uveitis, it is possible to have anterior chamber cells “spill over” into the anterior vitreous. These vitreous cells, like anterior vitreous cells, are inflammatory cells that arise from a breakdown of the blood-aqueous barrier. They are best visualized in a dilated eye at the slit lamp. Use the SUN grading scheme for vitreous cells (Table 1).6
Clinical examination of a patient with anterior uveitis should include visual acuity, slit lamp exam, IOP assessment and a dilated fundus exam.10 If IOP is elevated, gonioscopy may add valuable clues to the clinical course (acute vs chronic). The purpose of the dilated exam is to ensure the anatomical location of uveitis is confined to the anterior segment and that there are no posterior segment complications such as cystoid macular edema, cataracts (specifically posterior subcapsular cataracts) or glaucoma. If posterior uveitis or panuveitis is present, suspicion for underlying etiology increases and management changes. If you find that the anatomical location of the uveitis is isolated to the anterior chamber, determine whether the inflammation is granulomatous vs non-granulomatous based on clinical signs as discussed above.
Further, clinical signs, symptoms and history should enable classification of the clinical course (acute, chronic, or recurrent). For instance, a patient with acute AU will likely complain of sudden onset of redness, pain and light sensitivity, whereas a patient with chronic anterior uveitis may have few to no symptoms.
Based on the clinical course, clinical signs and pathophysiology of inflammation, narrow your differential underlying etiology to infectious vs non-infectious.
 |
| Fig. 2. Example of granulomatous or mutton-fat KP on the endothelium of a patient with sarcoidosis. |
Treatment
In general, the goals for treatment of AU are to eliminate ocular inflammation to preserve vision, relieve pain and prevent ocular complications, as well as identify any possible underlying etiology.
For acute AU, initial treatment includes topical corticosteroids and cycloplegic agents in an effort to reduce and/or eliminate intraocular inflammation. The dosing of topical corticosteroids often depends on the severity and clinical course of the anterior uveitis as well as the type of corticosteroid used.
Prednisolone acetate 1% is the most commonly prescribed corticosteroid used for treating AU, followed by dexamethasone 0.1%.1,4,11-13 For acute AU, the typical dosing is one drop every one to two hours while awake for a minimum of one week. Dosing for chronic AU may be less.
Difluprednate 0.05% emulsion is generally dosed QID and has been found to have similar efficacy to that of prednisolone acetate 1% when dosed eight times daily.11,14 The lesser dosing regimen of difluprednate 0.05% may offer better patient adherence, but has been shown to cause higher risk of steroid-induced IOP elevation compared with prednisolone acetate 1%.15
Loteprednol 0.5% is often reserved for patients in whom IOP spikes are a concern, but its efficacy is less than that of prednisolone acetate 1% or difluprednate 0.05%.16
Cycloplegia is generally used as an adjunctive therapy to corticosteroids. Cycloplegia decreases pain and photophobia by paralyzing the ciliary body and by preventing posterior synechiae.1,4,11-13 Immobilizing the ciliary body also helps to re-establish the blood-aqueous barrier. The dosing of the cycloplegic agent varies depending on the medication. Traditional treatment included homatropine 5% or scopolamine 0.25% or 0.5%, but due to their limited access it is becoming common to use atropine 1.0%. Cyclopentolate 1.0% is another topical cycloplegic option.
Although signs and symptoms may improve after initiation of topical corticosteroids and cycloplegia, continue treatment until inflammatory anterior chamber cells have resolved. This may take from one week to one month or more, depending on the underlying etiology. In rare cases, inflammatory cells may persist for years.
The typical follow-up for acute AU is five to seven days, at which time the anterior chamber cells should be reduced by at least 50% since the initial presentation.1,4,11 Patients should be re-examined weekly without altering corticosteroid and cycloplegia dosing until there are five or fewer cells per high-powered field as viewed by slit lamp. Visual acuity, slit lamp biomicroscopy, IOP and fundus examination should be performed at each follow-up visit. Additional testing may be indicated if ophthalmic complications arise, such as reduced vision or cystoid macular edema.
Once inflammatory cells of five or fewer per high-powered field are visible in the anterior chamber, prednisolone acetate 1% dosing may be reduced from every one hour to every two hours, provided patient symptoms have resolved.1,4,11-13 Follow-up visits may be extended to every two weeks and tapering steroids may continue, provided signs and symptoms do not increase.
Tapering Steroids in Anterior UveitisA reasonable tapering schedule of topical corticosteroids for anterior uveitis is one drop every two hours for two weeks, one drop QID for two weeks, one drop TID for two weeks, one drop BID for two weeks, one drop a day for two weeks, and then topical therapy should be discontinued.1,4,10 This schedule may need to be altered depending on the individual patient, past history, underlying etiologies, and complications and/or adverse events from the steroids. If the disease flares at any time during the follow-up process or inflammation increases, the corticosteroid dosing may need to be increased, followed by a slower taper.10-13 |
Underlying Etiologies
Although the majority of uveitis is undifferentiated or idiopathic (48% to 70%), infectious and non-infectious causes occur.17,18 Consider systemic etiologies when the AU is bilateral, recurrent, chronic or granulomatous in nature and when patients are younger than 15 years of age. Careful review of systems and lab testing are required to help narrow the differential diagnosis and ultimately identify the systemic condition.9 Failure to properly diagnose and treat systemic disease may result in ophthalmic treatment failure, ophthalmic complications, loss of vision, or even loss of life.
Uveitis-specific questionnaires—such as the Ocular Inflammation Disease Review of Systems Questionnaire—allow for a critical review of systems that guides the lab testing.4 In general, labs are withheld for initial acute episodes that are unilateral and non-granulomatous and if the uveitis responds to treatment.
If the AU is chronic, recurrent, bilateral, granulomatous and recalcitrant to treatment, laboratory testing is indicated; however, lab tests should be specific to the suspected underlying etiology (Table 2).10 Once the underlying etiology is identified, ocular management may change from topical therapy to disease-specific systemic treatment, especially for infectious conditions such as Lyme disease, syphilis, herpes and tuberculosis.
 |
Fig. 3. This patient has a fibrin membrane across the pupil with 4+ flare, 3+ cells and endothelial keratic precipitates. |
Non-infectious Etiologies
The most common non-infectious underlying etiology for AU is caused by a group of inflammatory disorders collectively termed the seronegative spondyloarthropathies, which account for up to 50% of acute and recurrent anterior uveitis.19
The seronegative spondyloarthropathies are negative for rheumatoid factor (RF) and anti-nuclear antibodies (ANA), but may be positive for HLA-B27, a class I major histocompatibility complex.19-23
The conditions associated with HLA-B27 seronegative spondyloarthropathies include ankylosing spondylitis, reactive arthritis syndrome, psoriatic arthritis and inflammatory bowel disease, including Crohn’s and ulcerative colitis. An estimated 50% of patients with acute anterior uveitis test positive for HLA-B27, and half of those patients go on to develop one of the seronegative spondyloarthropathies.24
Uveitis associated with HLA-B27 is typically recurrent unilateral, bilateral or alternating non-granulomatous AU and may have fine endothelial KPs.19-32 Symptoms associated with HLA-B27-positive acute AU are typically extreme and include eye pain, photophobia and intense injection of the bulbar conjunctiva.
Treatment includes topical corticosteroids and cycloplegia. For patients with non-infectious etiologies who don’t respond to topical therapy or for patients with recurrent AU, consider systemic treatment with oral corticosteroids or disease-modifying antirheumatic drugs such as methotrexate or hydroxychloroquine.
Other, less common non-infectious etiologies of AU include juvenile idiopathic arthritis (JIA), sarcoidosis, Behcet’s disease and systemic lupus erythematous (SLE).
• Juvenile idiopathic arthritis. JIA-related uveitis accounts for 20% to 40% of pediatric uveitis patients.25 JIA has many different subcategories, but the majority of JIA-associated uveitis patients have oligoarticular onset JIA (78% to 90%), while 7% to 14% have polyarticular JIA.10,26
The onset of JIA uveitis is typically insidious; many patients are asymptomatic with a white and quiet eye. Further, the arthritis often manifests before the uveitis is detected. The uveitis can become chronic, causing ocular complications such as cataracts, posterior synechia, band keratopathy, glaucoma and macular edema in 37.3% of patients with JIA-related AU.27
Because uveitis in JIA can present in a quiet eye, screening is recommended at three months for high-risk JIA patients, six months for moderate-risk JIA patients, and 12 months for low-risk JIA patients.26,28
Patients with JIA typically present with an acute recurrent unilateral or bilateral non-granulomatous AU.29 Work-up for children or adolescents suspected of JIA-related AU should include ANA, HLA-B27, and RF. A positive ANA increases the risk for AU, whereas a positive HLA-B27 increases the risk for developing ankylosing spondylitis later in life.
JIA-associated uveitis is often chronic, requiring long-term treatment. Systemic “steroid-sparing” therapeutic options include antimetabolites and other biologic agents.25
• Sarcoidosis. This is a multisystem disease of unknown origin that predominantly affects the lungs. Ocular involvement is present in up to 50% of patients.30-33 The hallmark sign of sarcoidosis is non-caseating granulomas caused by granulomatous inflammation. These are composed of epithelioid and giant cells that secrete angiotensin converting enzyme (ACE).
Anterior uveitis in sarcoidosis is typically bilateral, chronic and granulomatous with large mutton-fat KPs, TM inflammation and iris nodules.30-33 Up to 25% of patients with ocular sarcoidosis develop posterior segment involvement.30-33 Laboratory tests for patients suspected of sarcoidosis AU may include serum ACE levels, serum lysozyme, chest X-ray or CT, tissue biopsy or gallium scan.
In addition to topical ophthalmic treatment for anterior uveitis, systemic treatment may include oral corticosteroids.
• Systemic lupus erythematous. Like sarcoidosis, SLE is a multisystem autoimmune disease. While antibodies in the normal immune system protect against pathogens such as viruses and bacteria, ANA—a type III hypersensitivity reaction—attack cell nuclei triggering inflammation in patients with SLE.34,35 ANA levels are elevated in 97% of SLE patients. Although anterior uveitis can occur in SLE, it is most commonly associated with scleritis, episcleritis, secondary Sjögren’s syndrome, lupus retinopathy and choroidopathy.34,35 Anterior uveitis seldom occurs in isolation and is more commonly associated with scleritis or posterior uveitis.
• Behcet’s disease. This is a chronic, multisystem, relapsing inflammatory disorder of unknown etiology, characterized by the classic triad of oral and genital ulcers, ocular inflammation and skin lesions. It is associated with HLA-B51.36,37 The uveitis in patients with Behcet’s disease is typically bilateral, non-granulomatous anterior uveitis that may be acute, recurrent or chronic. Posterior uveitis may occur due to the increased risk of vasculitis associated with Behcet’s. Hypopyon is also a common finding, present in 19% to 31% of patients with AU and Behcet’s disease.38,39
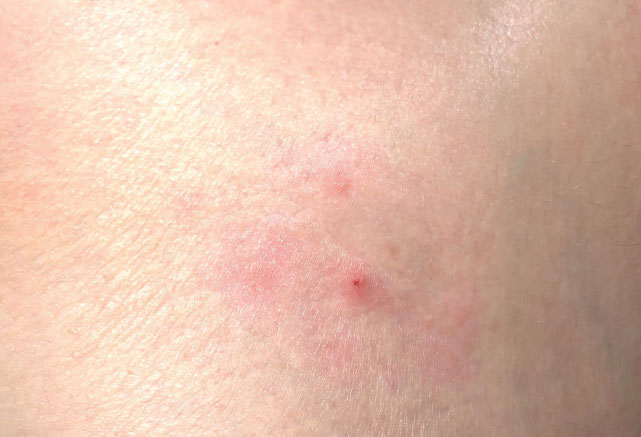 |
| Fig. 4. This patient has erythema migrans four days after a tick bite and was treated with 100mg doxycycline for 21 days. |
Infectious Etiologies
Consider infectious etiologies in patients with recurrent, chronic granulomatous anterior uveitis that fails to resolve with topical corticosteroids and whose review of systems suggests infection. Further, infectious etiologies should be ruled out prior to initiating any systemic corticosteroids or immunosuppressive agents.
Pathogens commonly causing anterior uveitis include bacteria and viruses. Fungal infections such as histoplasmosis and parasitic infections such as toxoplasmosis typically cause intermediate and posterior uveitis.
Bacterial Etiologies
Some of the more common bacterial etiologies of AU include syphilis, Lyme disease and tuberculosis (TB).
• Syphilis. This is a multisystem, chronic bacterial infection caused by the spirochete bacterium, Treponema pallidum. Syphilis accounts for 1% to 2% of uveitis cases but is considered the “Great Masquerader” due to the different stages it may progress through if left untreated.40-42 Primary syphilis is the first stage, which initially presents as erythematous papules at the inoculation site that later erode to a painless ulceration called a chancre, which may be present from two to six weeks.
If left untreated, primary syphilis leads to secondary syphilis. The latter occurs four to 10 weeks after infection, and the systemic treponemal load is largest in this stage. Secondary syphilis is characterized by a disseminated maculopapular rash on the palms of hands and soles of feet and lymphadenopathy. Constitutional symptoms may include high fever, malaise, headache, nausea, anorexia, hepatitis and meningitis. Approximately 10% of cases with secondary syphilis present with uveitis.40-42
Following secondary syphilis, patients progress to latent syphilis in which no systemic disease is apparent. Early latent syphilis occurs within one year of initial infection; late latent syphilis occurs after one year of infection. Most cases have been reported to remain at the latent stage, but 30% will progress to tertiary syphilis, the most common stage in syphilis for uveitis to develop.43-45
Tertiary syphilis may cause cardiovascular-syphilis, neuro-syphilis or benign-tertiary syphilis. AU in syphilis patients can occur in secondary, latent and tertiary stages of syphilis, but not present in primary syphilis. The uveitis may be unilateral or bilateral, granulomatous or non-granulomatous and with or without iris nodules, dilated iris vessels and iris atrophy.
Patients with syphilis may present with posterior uveitis such as diffuse or focal chorioretinitis, neuroretinitis, necrotizing retinitis, retinal vasculitis, intermediate uveitis or panuveitis.43-45
The diagnosis of syphilis is confirmed through serology testing and includes non-treponemal and treponemal tests. The non-treponemal tests include RPR and VDRL and treponemal tests include FTA-ABS or MHA-TP.
If the non-treponemal tests indicate active disease, determining the stage of syphilis will determine the systemic treatment protocol. Primary, secondary or early latent stage treatment includes a single intramuscular (IM) injection of penicillin.46 Late latent or tertiary stage requires weekly IM penicillin injections for a total of three doses, whereas neurosyphilis requires intravenous penicillin.46
Systemic treatment of syphilis-associated uveitis should occur in conjunction with ocular treatment with topical corticosteroids.
• Tuberculosis. This is a granulomatous infection caused by Mycobacterium tuberculosis. The classic presentation of an active TB infection includes chronic cough, fever, night sweats and weight loss. Granulomatous inflammation in TB causes granulomas to develop in the lungs, but granulomas may also develop on the iris, angle or choroid.
Uveitis may present in both active TB and in patients without systemic TB symptoms.47 The most common uveitis seen in TB is disseminated chorioretinitis, but it can also present as acute anterior uveitis, chronic granulomatous anterior uveitis, intermediate uveitis, vitritis or endophthalmitis.48-52
Making the diagnosis of TB requires lab tests. The purified protein derivative (PPD) skin test can identify past exposure but does not indicate if the disease is active. Chest CT/X-ray and sputum cultures are performed to determine if the infection is active. Blood tests, called interferon-gamma release assays, indicate TB infection.
• Lyme disease. This is a multisystem disorder caused by the spirochete Borrelia bergdorferi infection, which is transmitted via tick bites.
There are three stages of Lyme disease: early, disseminated and persistent. In the early stage of the disease, 60% to 80% of patients present with erythema migrans rash (Figure 4) that may take a bull’s-eye pattern at the site of the tick bite within two to 28 days after the bite.53 Associated symptoms in the early stage may include fever, malaise, fatigue, arthralgia and myalgia.
All forms of uveitis may be present in the later stages of the disease (weeks to months after the initial infection), including AU, intermediate uveitis, posterior uveitis, neuroretinitis, retinal vasculitis, choroiditis and panuveitis.54-58
If Lyme disease is suspected, immunofluorescence assay (IFA) or enzyme immunoassay (EIA) are performed first. If either test is positive or equivocal, IgM and IgG Western blot series are performed if signs or symptoms are ≤30 days. IgG Western blot is performed if signs or symptoms are >30 days.59
Doxycycline 100mg every 12 hours for 10 to 21 days is the recommended treatment for non-pregnant adults. Amoxicillin is 500mg TID for 14 to 21 days for pregnant adults and in children 50mg/kg divided into three doses per day for 14 to 21 days.59 Ocular treatment includes topical steroids in conjunction with systemic treatment.
 |
Viral Etiologies
The most common infectious underlying etiology of anterior uveitis is caused by viruses—most commonly herpes simplex (HSV), varicella zoster virus (VZV) and cytomegalovirus (CMV). The diagnosis of HSV anterior uveitis is often made based mainly on clinical features—50% to 90% of cases have elevated IOP, iris atrophy, KPs, and unilateral presentation.60
Herpesvirus 1 (HSV-1) is implicated in anterior uveitis that is granulomatous, unilateral, and often with increased IOP due to concomitant trabeculitis.60 Although HSV-associated AU is granulomatous, the KPs are usually fine and medium sized vs the mutton-fat appearance of other granulomatous etiologies.60 HSV anterior uveitis recurs an estimated 71% of the time, which increases the likelihood of iris atrophy.61-63
Herpesvirus 3—varicella zoster virus (VZV)—causes chickenpox. Following the initial infection, VZV remains dormant in neural ganglia. When VZV is reactivated, it causes herpes zoster (HZV), which typically manifests with unilateral pain in a dermatomal distribution accompanied by a maculopapular vesicular rash. When reactivated along the trigeminal nerve, it is termed herpes zoster ophthalmicus.63-67
Treat HSV- or VZV-associated AU with topical corticosteroids. Because chronic and/or recurrent AU is common, concomitant oral antiviral medication is often also required. Acyclovir 400mg (800mg for VZV) five times per day, valacyclovir 500mg (1,000mg for VZV) three times per day or famciclovir 500mg three times per day are all acceptable dosages to effectively treat patients with active herpes infection and ocular inflammation.60-68 Antiviral coverage can be reduced to two times per day for acyclovir or one time per day for valacyclovir or famciclovir once the ocular inflammation shows signs of reduction and the patient’s corticosteroid therapy has been tapered to one drop three times per day.60-68
Herpesvirus 5—cytomegalovirus (CMV)—is ubiquitous in humans and can cause major morbidity and mortality especially in immunocompromised patients; for instance, CMV retinitis is the most common ocular manifestation seen in patients with HIV/AIDS. CMV has been recognized as a cause of AU in patients who are HIV negative, accounting for 22.8% of cases associated with AU and raised IOP.69 CMV-associated AU in immunocompetent individuals may present with unilateral ocular hypertension, chronic or recurrent uveitis with patchy or diffuse iris atrophy, few fine KPs and mild anterior chamber cells. CMV-associated AU is thought to be the cause of Posner-Schlossman syndrome.69 Treatment for CMV-associated AU includes topical ganciclovir as a first-line treatment due to good tolerability and minimal side effects, in conjunction with topical corticosteroids.60,69,70
Anterior uveitis is a common condition that all primary eye care providers encounter. To effectively manage AU, the clinician must know not just the signs and symptoms associated with AU, but also the underlying etiologies that often cause it. Narrowing the etiology first requires identifying the AU as acute, chronic or recurrent. Past and current clinical signs and symptoms often guide this determination.
Further, one must know the different types of clinical signs associated with granulomatous vs non-granulomatous inflammation. Based on laterality (unilateral vs. bilateral vs alternating), clinical course (acute, chronic or recurrent), type of inflammation (granulomatous vs. non-granulomatous) and review of systems, the clinician should be able to differentiate any underlying etiology as infectious vs non-infectious. This will then allow the clinician to obtain any necessary laboratory tests to help support or confirm the suspected underlying etiology.
Once the underlying etiology is determined, a more targeted treatment of the ocular inflammation can be initiated through concomitant systemic management.
Dr. Opitz is an associate professor in the Department of Clinical Education at the Illinois College of Optometry and is the senior director of Ophthalmology Services and Practice Development for the Illinois Eye Institute.
| 1. Huang JJ, Gau PA. Ocular Inflammatory Disease and Uveitis Manual Diagnosis and Treatment. Philadelphia: Lippincott Williams & Wilkins; 2010:1-9. 2. Snell RS, Lemp MA. The eyeball. In: Snell RS, Lemp MA, eds. Clinical Anatomy of the Eye. 2nd ed. Malden, MA: Blackwell Science; 1998:140-156. 3. Rao NA, Forster DJ. Basic principles. In: Podos SM, Yanoff M, editors. The Uvea, Uveitis, and Intraocular Neoplasms. Vol. 2. New York: Gower Medical Publications; 1992:1-17. 4. Foster CS, Vitale AT. Diagnosis and Treatment of Uveitis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2013:20-32. 5. Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58(1):11-9. 6. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509-16. 7. Williams GT, Williams WJ. Granulomatous inflammation—a review. J Clin Pathol. 1983;36(7):723-33. 8. Herbort CP. Appraisal, workup and diagnosis of anterior uveitis: a practical approach. Middle East Afr J Ophthalmol. 2009;16(4):159-67. 9. Yanoff M, Duker JS. Ophthalmology. 2nd ed. St Louis: Mosby; 2004. 10. Harthan JS, Opitz DL, Fromstein SR, Morettin CE. Diagnosis and treatment of anterior uveitis: optometric management. Clin Optom (Auckl). 2016 Mar 31;8:23-35. 11. Foster CS, Davanzo R, Flynn TE, et al. Durezol (difluprednate ophthalmic emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmocol Ther. 2010;26(5):475-83. 12. Freeman WR, Green RL, Smith RE. Echographic localization of corticosteroids after periocular injection. Am J Ophthalmol. 1987;103(3 Pt 1):281-8. 13. Smith RE, Nozik RA. Uveitis: A Clinical Approach to Diagnosis and Management. Baltimore: Williams & Wilkins; 1989:51-76. 14. Sheppard JD, Toyos MM, Kempeen JH, Foster CS. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Invest Ophthalmol Vis Sci. 2014;55(5):2993-3002. 15. Kusne Y, Kang P, Fintelmann RE. A retrospective analysis of intraocular pressure changes after cataract surgery with the use of prednisolone acetate 1% versus difluprednate 0.05%. Clin Ophthalmol. 2016 Nov 23;10:2329-36. 16. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999;127(5):537-44. 17. Gutteridge IF, Hall AJ. Acute anterior uveitis in primary care. Clin Exp Optom. 2007;90(2):70-82. 18. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491-500. 19. Rosenbaum JT. Characterization of uveitis associated with spondyloarthritis. J Rheumatol. 1989;16(6):792-6. 20. Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25-31. 21. Rudwaleit M, Landewé R, van der Heijde D, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68(6):770-6. 22. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777-83. 23. Rosenbaum JT. Characterization of uveitis associated with spondyloarthritis. J Rheumatol. 1989;16(6):792-6. 24. Wakefield D, Chang JH, Amjadi S, et al. What is new HLA-B27 acute anterior uveitis? Ocul Immunol Inflamm. 2011;19(2):139-44. 25. Rabinovich CE. Treatment of juvenile idiopathic arthritis-associated uveitis: challenges and update. Curr Opin Rheumatol. 2011;23(5):432-6. 26. Hofer M, Southwood TR. Classification of childhood arthritis. Best Pract Res Clin Rheumatol. 2002;16(3):379-96. 27. Sabri K, Saurenmann RK, Silverman ED, Levin AV. Course, complications, and outcome of juvenile arthritis-related uveitis. J AAPOS. 2008;12:539–545. 28. Cassidy J, Kivlin J, Lindsley C, Nocton James. Ophthalmic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 2006;117(5):1843–1845. 29. Burgos-Vargas R, Pacheco-Tena C, Vázquez-Mellado J. The juvenile-onset spondyloarthritides: rationale for clinical evaluation. Best Pract Res Clin Rheumatol. 2002;16(4):551-72. 30. Birnbaum AD, Oh FS, Chakrabarti A, et al. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol. 2011;129(4):409-13. 31. Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102(3):297-301. 32. Karma A, Huhti E, Poukkula A. Course and outcome of ocular sarcoidosis. Am J Ophthalmol. 1988;106(4):467-72. 33. Crick RP, Hoyle C, Smellie H. The eyes in sarcoidosis. Br J Ophthalmol. 1961;45(7):461-81. 34. Gold DH, Morris DA, Henkind P. Ocular findings in systemic lupus erythematosus. Br J Ophthalmol. 1972;56(11):800-4. 35. Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. Br J Ophthalmol. 2016;100(1):135-41. 36. Ando K, Fujino Y, Hijikata K, et al. Epidemiological features and visual prognosis of Behcet’s disease. Jpn J Ophthalmol. 1999;43(4):312-7. 37. Nussenblatt RB. Uveitis in Behcet’s disease. Int Rev Immunol. 1997;14(1):67-79. 38. Mishima S, Masuda K, Izawa Y, et al. The eighth Frederick H. Verhoeff Lecture, presented by Saiichi Mishima, MD, Behcet’s disease in Japan: ophthalmologic aspects. Trans Am Ophthalmol Soc. 1979;77:225-279. 39. Baer JC, Raizman MB, Foster CS. Ocular Behçet’s disease in the United States. Clinical presentation and visual outcome in 29 patients. In: Masahiko U, Shigeaki O, Koki A, eds. Proceedings of the Fifth International Symposium on the Immunology and Immunopathology of the Eye; Tokyo, New York: Elsevier Science; 1990:383. 40. Barile GR, Flynn TE. Syphilis exposure in patients with uveitis. Ophthalmology. 1997;104(10):1605-9. 41. Margo CE, Hamed LM. Ocular syphilis. Surv Ophthalmol. 1992;37(3):203-20. 42. Deschenes J, Seamone CD, Baines MG. Acquired ocular syphilis: diagnosis and treatment. Ann Ophthalmol. 1992;24(4):134-8. 43. Tamesis RR, Foster CS. Ocular syphilis. Ophthalmology. 1990;97(10):1281-7. 44. Mendelsohn AD, Jampol LM. Syphilitic retinitis. A cause of necrotizing retinitis. Retina. 1984;4(4):221-4. 45. McLeish WM, Pulido JS, Holland S, et al. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology. 1990;97(2):196-203. 46. 2015 Sexually Transmitted Diseases Treatment Guidelines. Centers for Disease Control and Prevention website. July 27, 2016. www.cdc.gov/std/tg2015/syphilis.htm. Accessed September 3, 2018. 47. Suzuki J, Oh I, Kezuka T, Sakai J, Goto H. Comparison of patients with ocular tuberculosis in the 1990s and the 2000s. Jpn J Ophthalmol. 2018;54(1):19-23. 48. Cutrufello NJ, Karakousis PC, Fishler J, Albini TA. Intraocular tuberculosis. Ocul Immunol Inflamm. 2010;18(4):281-91. 49. Gupta A, Bansal R, Gupta V, et al. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149(4):562-70. 50. Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):7-13. 51. Madge SN, Prabhakaran VC, Shome D, et al. Orbital tuberculosis: a review of the literature. Orbit. 2008;27(4):267-77. 52. Rosen PH, Spalton DJ, Graham EM. Intraocular tuberculosis. Eye (Lond) 1990;4(pt 3):486-92. 53. Guidelines for laboratory evaluation in the diagnosis of Lyme disease. American College of Physicians. Ann Intern Med. 1997;127(12):1106-8. 54. Isogai E, Isogai H, Kotake S, et al. Detection of antibodies against Borrelia burgdorferi in patients with uveitis. Am J Ophthalmol. 1991;112(1):23-30. 55. Karma A, Viljanen M, Pirttila T, et al. Ocular Lyme borreliosis. Duodecim. 1993;109(1):35-42. 56. Karma A, Seppala I, Mikkila H, et al. Diagnosis and clinical characteristics of ocular Lyme borreliosis. Am J Ophthalmol. 1995;119(2):127-35. 57. Mikkila H, Karma A, Viljanen M, Seppala I. The laboratory diagnosis of ocular Lyme borreliosis. Graefes Arch Clin Exp Ophthalmol. 1999 Mar;237(3):225-30. 58. Flach AJ, Lavoie PE. Episcleritis, conjunctivitis, and keratitis as ocular manifestations of Lyme disease. Ophthalmology. 1990;97(8):973-5. 59. Lyme Disease – Health Care Providers – Diagnosis, Treatment and Testing. Centers for Disease Control and Prevention website. December 21, 2018. www.cdc.gov/lyme/healthcare/index.html. Accessed September 3, 2018. 60. Jap A, Chee SP. Viral anterior uveitis. Curr Opin Ophthalmol. 2011;22(6):483-8. 61. North RD, Pavan-Langston D, Geary P. Herpes simplex virus types 1 and 2: therapeutic response to antiviral drugs. Arch Ophthalmol. 1976;94(6):1019-21. 62. Pavan-Langston D, Grene B. Herpes simplex ocular disease. Compr Ther. 1984;10(6):30-6. 63. Pavan-Langston D. Herpes simplex and herpes zoster keratouveitis: diagnosis and management. Bull N Y Acad Med. 1977;53(8):731-48. 64. Pavan-Langston D. Varicella-zoster ophthalmicus. Int Ophthalmol Clin. 1975;15(4):171-85. 65. Pavan-Langston D. Ocular viral infections. Med Clin North Am. 1983;67(5):973-90. 66. Pavan-Langston D. Herpes zoster ophthalmicus. Neurology. 1995;45(12 Suppl 8):S50-1. 67. Womack LW, Liesegang TJ. Complications of herpes zoster ophthalmicus. Arch Ophthalmol. 1983;101(1):42-5. 68. A controlled trial of oral acyclovir for iridocyclitis caused by herpes simplex virus. The Herpetic Eye Disease Study Group. Arch Ophthalmol. 1996;114(9):1065-72. 69. Chee SP, Bacsal K, Jap A, et al. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145(5):834-40. 70. Chee SP, Jap A. Cytomegalovirus anterior uveitis: outcome of treatment. Br J Ophthalmol. 2010;94:1648-52. |
