Eyelids & Ocular SurfaceThe November 2024 Review of Optometry issue showcases five articles on eyelid & ocular surface conditions, from MGD to scleritis. Read about what to do when dry eye patients aren’t responding to treatment, corneal AEs caused by systemic meds, innovative MGD treatments, managing ocular cellulitis and more. Check out the other articles featured in this issue: |
All sorts of medications cause ophthalmic side effects and can affect specific anatomical structures of the eye. A common example is hydroxychloroquine, which causes a dose-related maculopathy when it accumulates in the maculae.1 Medications can similarly accumulate in the cornea and it’s our responsibility as clinicians to be vigilant and responsive when it happens.
Drug-induced iatrogenic deposits are often asymptomatic but can cause visible opacities that typically affect specific layers or regions of the cornea. Although meds may reach the cornea via tears, aqueous humor or perilimbal vasculature, the exact pathophysiology and mechanisms behind layer-specific deposits remain poorly understood.1 While some deposits are well-documented in the literature, others are scarcely mentioned in case reports.
When conducting a comprehensive eye examination, reviewing a patient’s ocular and systemic medications is essential. At times, patients may present with corneal findings as a result of medication side effects. If a medication side effect is causing signs or symptoms, it is our responsibility as eyecare providers to collaborate with other specialists to ensure the best care for our patients.
This article will review medications that deposit in all layers of the cornea. Some are incidental findings that do not cause symptoms, while others may be visually bothersome and require drug discontinuation. Some discussed agents affect a single layer of the cornea, with others affecting multiple layers.
Vortex Keratopathy
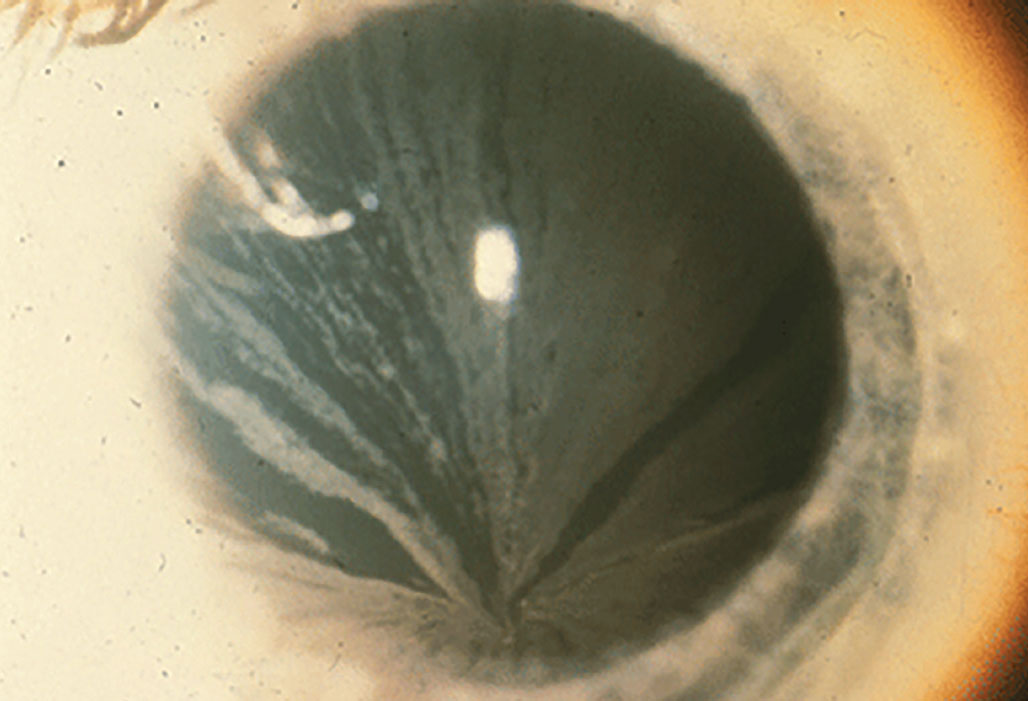
The most common drug-related alteration in the corneal epithelium is deposition of phospholipids in a vortex or whorl-like pattern—otherwise known as vortex keratopathy or corneal verticillata. This whorl-like pattern is typically induced by cationic amphiphilic drugs and follows the natural centripetal migration of the corneal epithelium from the limbus to the center.
 |
|
Vortex keratopathy typically follows the natural centripetal migration of the corneal epithelium from limbus to center. Photo: Christine Sindt, OD. Click image to enlarge. |
Vortex keratopathy typically begins with punctate deposits that are gray, blue, brown or golden in color. As more deposits accumulate, they begin to form the characteristic whorl. The pattern appears most commonly in the inferior cornea but can also be found centrally.2 This presentation rarely has an effect on visual acuity, but some individuals do report halos around lights or glare. The keratopathy is usually reversible three to 20 months upon discontinuing treatment of the offending agent.3
The most well-researched cause of vortex keratopathy is amiodarone; however, a variety of other pharmaceuticals can cause this corneal change, including aminoquinolones (hydroxychloroquine, chloroquine, amodiaquine), atovaquone, clofazimine, gentamicin (subconjunctival), gold, ibuprofen, indomethacin, mepacrine, monobenzone (topical ointment), naproxen, perhexiline maleate, phenothiazines, suramin, tamoxifen and tilorone hydrochloride.2,3 These medications are commonly amphiphilic, allowing them to penetrate lysosomes in corneal cells and deposit lipids into the area.2 Especially relevant to eyecare providers, topical rho-kinase inhibitors used to decrease intraocular pressure have also been shown to cause corneal deposition in a whorl-like pattern.4 An alternate presentation of whorl keratopathy is due to Fabry disease, a lysosomal storage disease. Since there are systemic implications of Fabry disease, it is essential to distinguish between Fabry and medication-induced corneal changes.
 |
|
Lipid keratopathy occurs commonly from amphiphilic medications that can penetrate lysosomes in corneal cells and deposit lipids. Photo: Christine Sindt, OD. Click image to enlarge. |
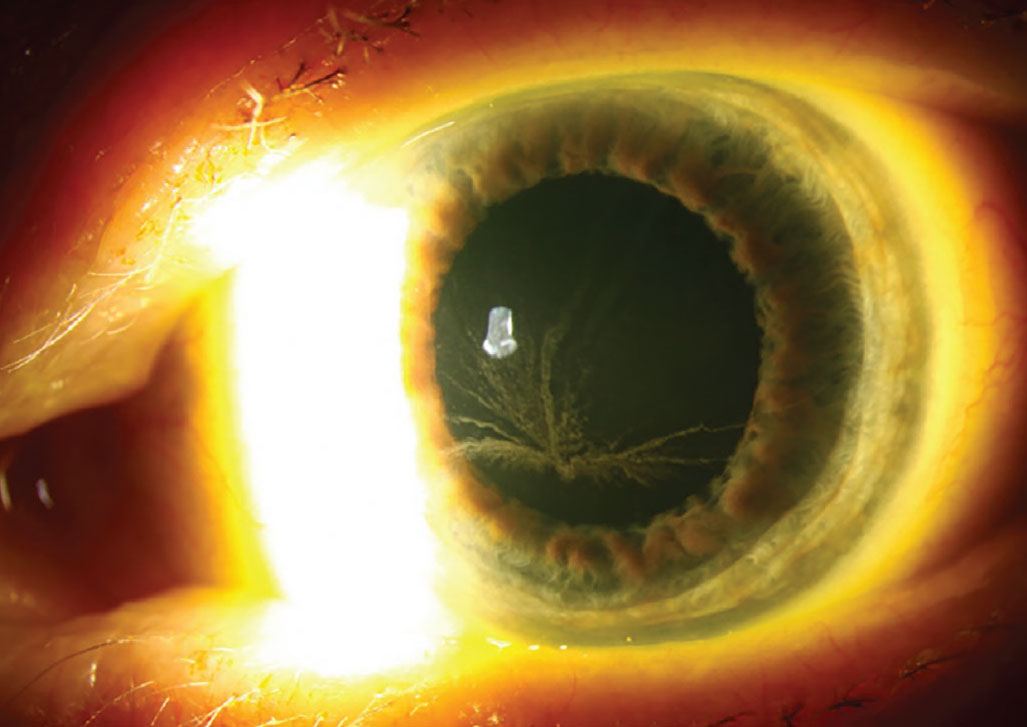
Fabry disease is caused by a deficiency of the enzyme α-galactosidase A, leading to the inability to break down globotriaosylceramide. As a result, globotriaosylceramide accumulates in lysosomes throughout the body, with the disorder presenting in two forms: classic and atypical. The classic form is associated with widespread dysfunction, affecting the skin, heart, kidneys, brain, blood vessels, eyes and nervous system. In contrast, atypical Fabry disease often manifests as cardiac dysfunction with minimal or no ocular involvement. Fabry disease is an X-linked recessive disorder mainly affecting men and occurs in approximately one in 40,000 to one in 60,000 individuals.5 The disease demonstrates approximately equal distribution amongst all racial and ethnic groups. The more prominent and classic presentation of corneal disease involves the presence of cornea verticillata, which can be observed as opacified, snaking lines originating from the central cornea.6
Verticillata occur early in the course of Fabry disease and are seen in up to 70% of these patients. In an otherwise asymptomatic patient, their presence requires further evaluation. Hyperreflective intracellular inclusions at the level of the basal epithelial cells or the epithelial basement membrane are examples of corneal manifestations. Bowman’s layer may be involved, causing a distorted and uneven appearance. Women may be affected differently, in which the inclusions were finer and more diffuse.7 The central and peripheral cornea are similarly affected, with opacities emerging in a vortex-like pattern; this results from the centripetal migration of deposit-laden limbal stem cells as the corneal epithelium undertakes natural growth and repair.
 |
|
In Fabry disease, bilateral corneal diffuse yellow epithelial haziness eventually becomes dense bronze to cream-colored streaks arranged in a vortex or star-shaped pattern (whorl-like or verticillata). Photo: Leonid Skorin Jr., OD, DO. Click image to enlarge. |
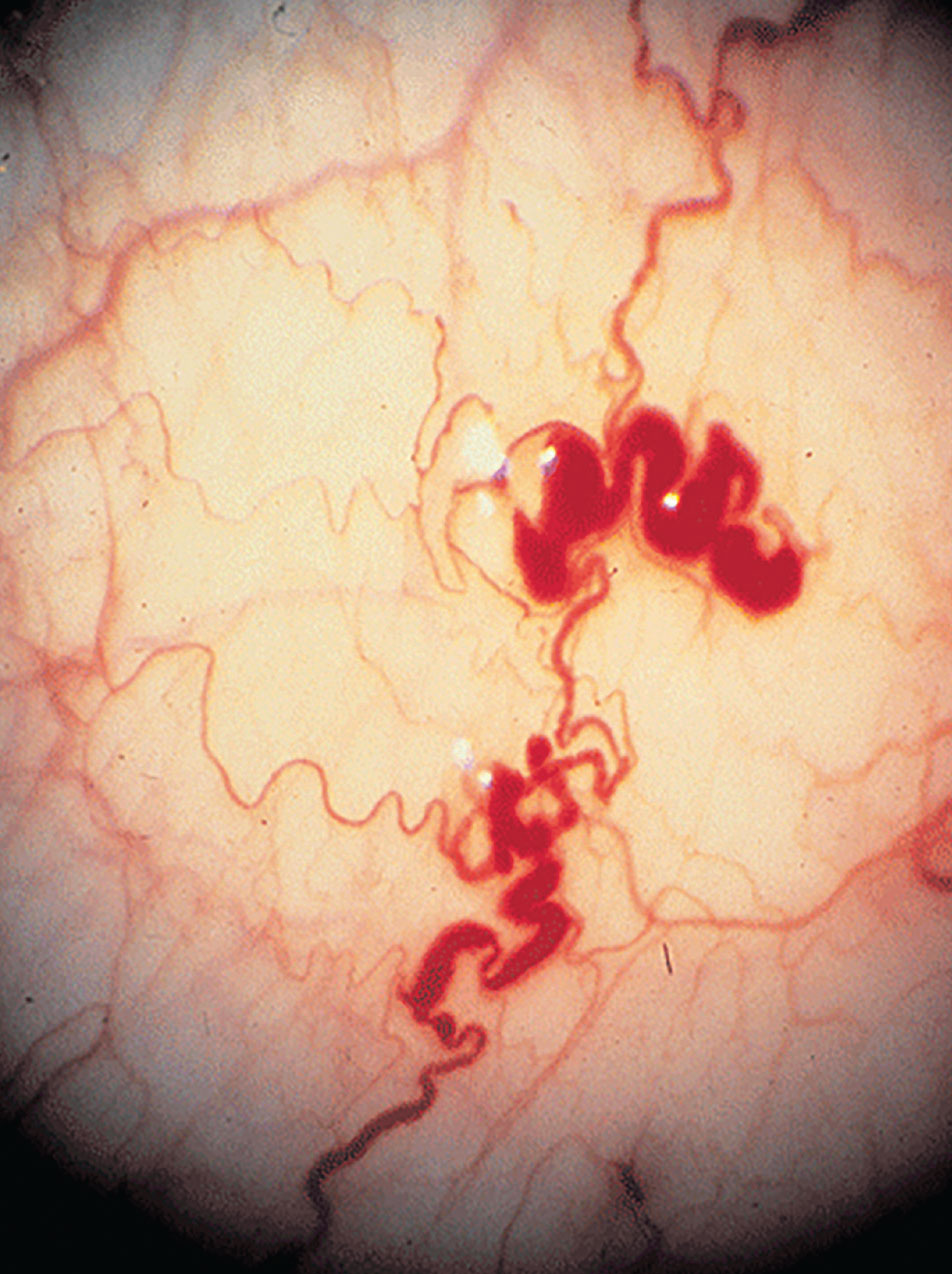
Conjunctival involvement with Fabry disease is similar in men and women with the appearance of round intracellular inclusions. In addition, vascular abnormalities include vessel tortuosity, microaneurysms and general dilation. It has been hypothesized that the anomalies in the vessels are caused by the vascular endothelium’s aberrant storage of globotriaosylceramide, which weakens and breaks down vessel walls; this hypothesis is similar for retinal vascular disease.6,8 The most frequent finding, though, is retinal vessel tortuosity.6 The lysosomal inclusions occur in endothelial cells and pericytes of retinal vessels.8 Anterior capsular or posterior subcapsular cataracts are the most widespread manifestations, although there have been reports of retinal artery occlusions, optic disc edema, optic atrophy, lid edema, dry eye syndrome and visual field defects.6,8
 |
|
The small vessels of the conjunctiva in Fabry disease often show aneurysmal dilations, tortuosity and kinking. Photo: Leonid Skorin Jr., OD, DO. Click image to enlarge. |
Neuropathic pain with a characteristic onset in childhood, dermatologic manifestations such as telangiectasias and angiokeratomas, renal failure, strokes and deafness are non-ocular manifestations of Fabry disease. Individuals do not tend to live beyond the fourth or fifth decade of life. A positive family history is the single known risk factor for Fabry disease, with no suggested environmental risk factors. It is important to understand the manifestations of Fabry disease to properly distinguish if vortex keratopathy may result from the condition or if systemic medications need to be considered as a cause.
Amiodarone is a class three antiarrhythmic used to treat ventricular arrhythmias. It works through blockage of potassium ion channels (prolonging repolarization), blocking sodium ion channels, and antagonizing alpha- and beta-adrenergic receptors which can lead to an irregular heartbeat; this is achieved by acting directly on the heart tissue to slow nerve impulses, helping to maintain a normal heart rhythm.
Amiodarone causes vortex keratopathy in 98% of those who take the medication at doses starting at 200mg to 300mg per day, with keratopathy typically being bilateral and usually symmetric.1,3 Increased duration of treatment and higher doses (greater than 400mg/day) is associated with more severe and more visually significant keratopathy.2 Individuals may describe halos, photophobia and irritation.1 Since visual acuity is rarely affected, the risk of discontinuing the medication generally outweighs the benefit of treating the keratopathy. In symptomatic individuals, consultation with cardiology is indicated.
Used to treat malaria and rheumatoid arthritis, the aminoquinolone class includes hydroxychloroquine, chloroquine and amodiaquine. Aminoquinolones act during the blood phase of the disease, targeting parasites once they enter the bloodstream and invade red blood cells. The most significant and well-known ocular complication with aminoquinolones is bull’s eye maculopathy, but the class is also associated with corneal deposition and vortex keratopathy.2 Unlike maculopathy, the corneal deposition is reversible if the drug is discontinued. Chloroquine is more closely associated with keratopathy than hydroxychloroquine in the aminoquinolone class of medications, and individuals with hydroxychloroquine-related retinopathy are also more likely to show corneal deposition.2
Suramin, a reverse transcriptase inhibitor used to treat AIDS, metastatic prostate cancer and infections caused by parasites, including African trypanosomiasis and onchocerciasis, is associated with vortex keratopathy in high doses. There have also been some reports of punctate keratitis and epithelial erosions, although these are rare presentations.3 Like most other drug-related epithelial changes, both are reversible upon discontinuation of the drug.2
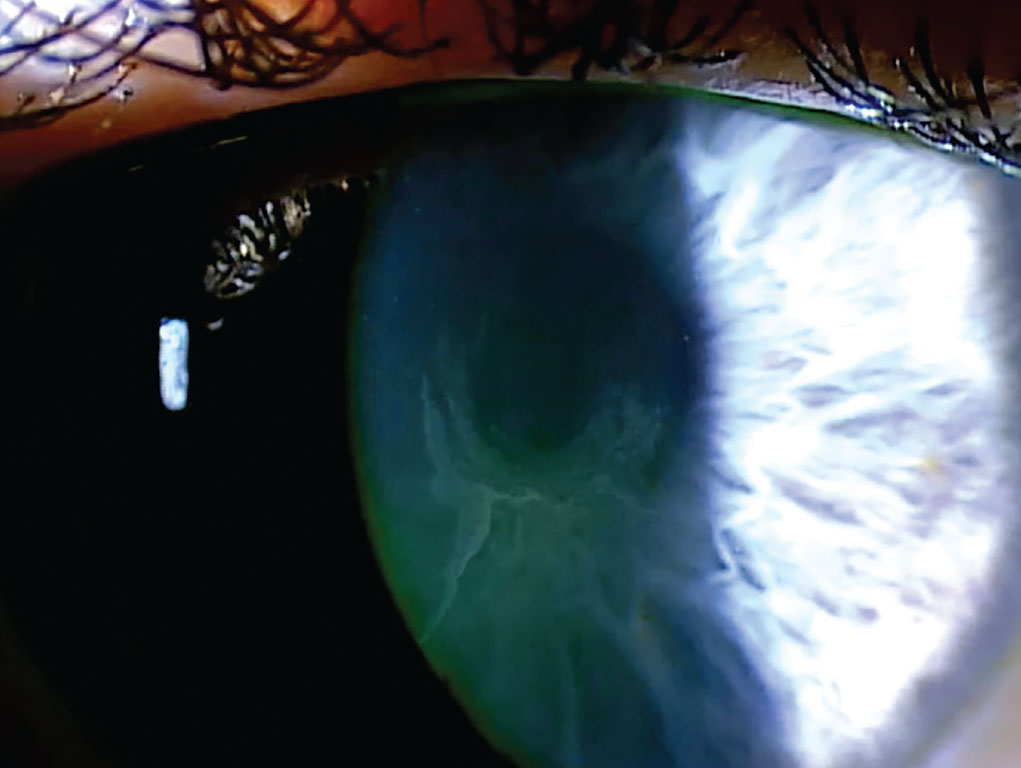 |
|
Vortex keratopathy is the most common drug-related alteration to the corneal epithelium, creating a whorl-like pattern. Photo: Mile Brujic, OD. Click image to enlarge. |
Non-steroidal anti-inflammatory drugs (NSAIDs) are common pharmaceuticals taken to reduce fever, pain and inflammation. NSAIDs work by inhibiting the enzyme cyclooxygenase, an agent involved in the production of prostaglandins responsible for pain, fever and inflammation. NSAIDs including naproxen, ibuprofen and indomethacin have also been associated with vortex keratopathy and deposits in Bowman’s membrane. Indomethacin is the most common NSAID to cause vortex keratopathy, which is reversible weeks after cessation of treatment.
Keratopathy due to naproxen and ibuprofen present with parallel lines in the superficial cornea in a fingerprint pattern, followed by the progression of a clear-cut corneal verticillate pattern.9,10 Corneal deposits occur quickly, within days of NSAID treatment, and particularly at high doses, such as 1200mg/day for ibuprofen.1 NSAID-associated corneal deposits are rare and the explicit reason why a subset of patients develop these deposits is not known.
Epithelial Microcysts
Antineoplastic agents are chemotherapeutic drugs designed to control or destroy cancer cells. These medications are cytotoxic and inhibit or prevent cell function, but they tend to be more harmful to rapidly dividing cells than to resting cells. Antineoplastic medications have been frequently associated with epithelial microcysts and punctate keratopathy. One example is cytarabine, used to treat acute myelogenous leukemia.3 Since cytarabine inhibits DNA synthesis, the rapidly dividing epithelial cells are targeted. Typical symptoms include blurred vision, glare, tearing and foreign body sensation. Symptoms can resolve without treatment, but some practitioners find success treating prophylactically with topical steroids to minimize formation of microcysts.2
Antibody-drug conjugates (ADCs) are a fairly new type of antineoplastic agent created through binding a monoclonal antibody to a cytotoxic drug. ADCs are a promising cancer treatment modality that allow for the selective delivery of highly cytotoxic agents directly to tumors. The monoclonal antibodies found in ADCs are also used to treat autoimmune disorders such as Crohn’s disease and rheumatoid arthritis. Numerous ADCs have corneal adverse effects, and these microcystic changes are the most common ocular adverse events seen. It is hypothesized that corneal toxicity is a result of off-target delivery and deconjugation of the cytotoxic drug.1
Belantamab mafodotin is an ADC used to treat multiple myeloma, with the agent showing an association with epithelial microcysts, decreased tear break-up time and decreased corneal sensitivity. Small intraepithelial microcyst-like epithelial changes may be present along with superficial punctate keratopathy.11 Generally, individuals are symptomatic and visual acuity can be affected.12 After drug discontinuation, the epithelial microcysts do resolve, but it is unclear if corneal innervation and sensitivity return to normal.
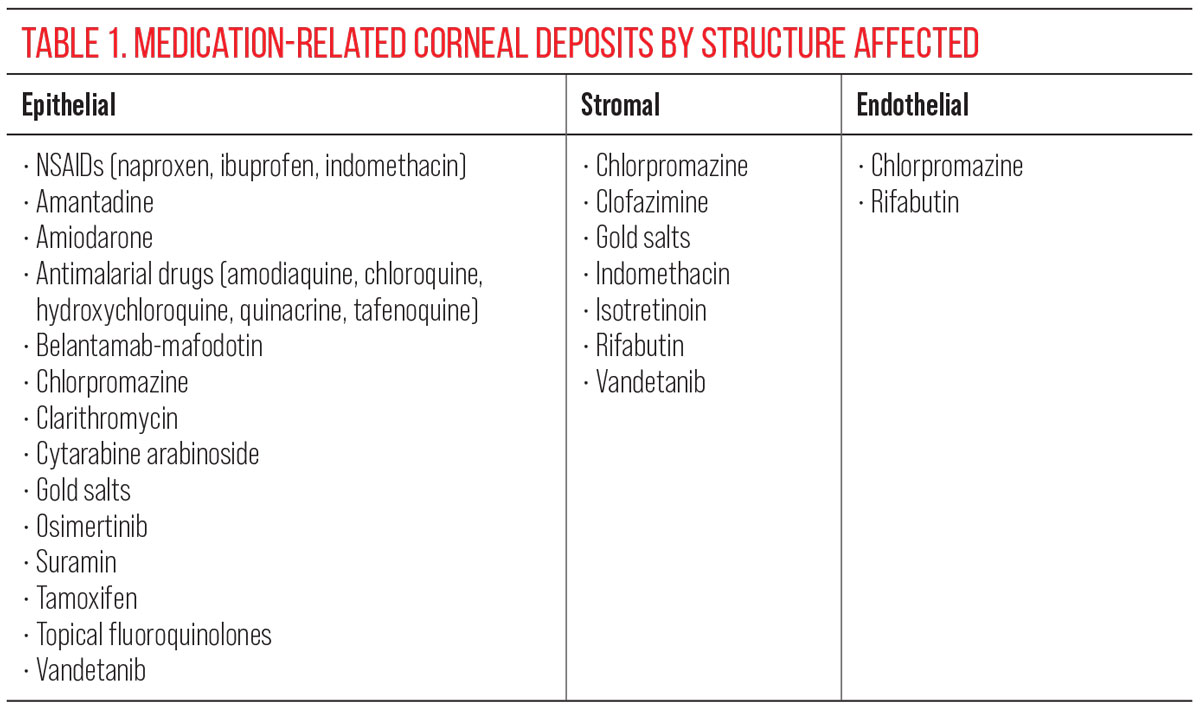 |
| Click image to enlarge. |
Two ADCs, Elahere (mirvetuximab soravtansine-gynx, AbbVie) and Tivdak (tisotumab vedotin-tftv, Pfizer and Genmab), are agents used to treat gynecologic cancers that also have ocular implications. Elahere is given by infusion every three weeks used to treat ovarian, fallopian tube or primary peritoneal cancer that is platinum-resistant and folate receptor alpha (FRα)-positive. Elahere binds to FRα on cancer cells and releases a chemotherapy drug to kill the cells. Ocular side effects of Elehare include blurred vision, ocular pain, tearing, foreign body sensation and photophobia.13 Fine corneal subepithelial cystic opacities primarily involve the corneal periphery and migrate toward the center. With a short course of topical steroids and lubricants, the cornea can clear and visual acuity can fully recover.13
Tivdak is an injection used to treat recurrent or metastatic cervical cancer. It is given once every three weeks and can cause severe ocular toxicities resulting in changes in vision, including severe vision loss and corneal ulceration. An eye exam is recommended prior to the initiation of Tivdak, prior to every cycle for the first nine cycles and as clinically indicated. Tivdak targets tissue factor, which is often expressed on cervical cancer cells, and delivers a cytotoxic agent directly to the tumor cells.
Ocular adverse reactions occurred in 55% of patients with cervical cancer treated with Tivdak across clinical trials. The most common adverse effects observed were conjunctivitis (32%), dry eye (24%), keratopathy (17%) and blepharitis (5%).14 Grade 3 ocular adverse reactions occurred in 3.3% of patients, including severe ulcerative keratitis in 1.2% of patients.14 Nine patients (2.1%) experienced ulcerative keratitis, including one with perforation requiring corneal transplantation, six (1.4%) with conjunctival ulcer, four (0.9%) with corneal erosion, two (0.5%) with conjunctival erosion and two (0.5%) with symblepharon.14
Corneal Infiltrates
Dupilumab is a Il4 receptor alpha agonist biologic agent used to treat allergic conditions of atopic dermatitis, chronic sinusitis and eczema and has been associated with limbal infiltrates and corneal ulceration.15 These infiltrates are typically sterile and can be treated with prophylactic topical antibiotics. Topical corticosteroids should be introduced after the epithelial defect has resolved to minimize corneal scarring. It is also necessary to consult with a dermatologist and consider discontinuation of dupilumab if individuals experience an adverse event. In at least two cases, a dupilumab-related ulcer progressed to corneal perforation, stressing the importance of monitoring these patients closely.16 Dupilumab is also linked with conjunctivitis.1
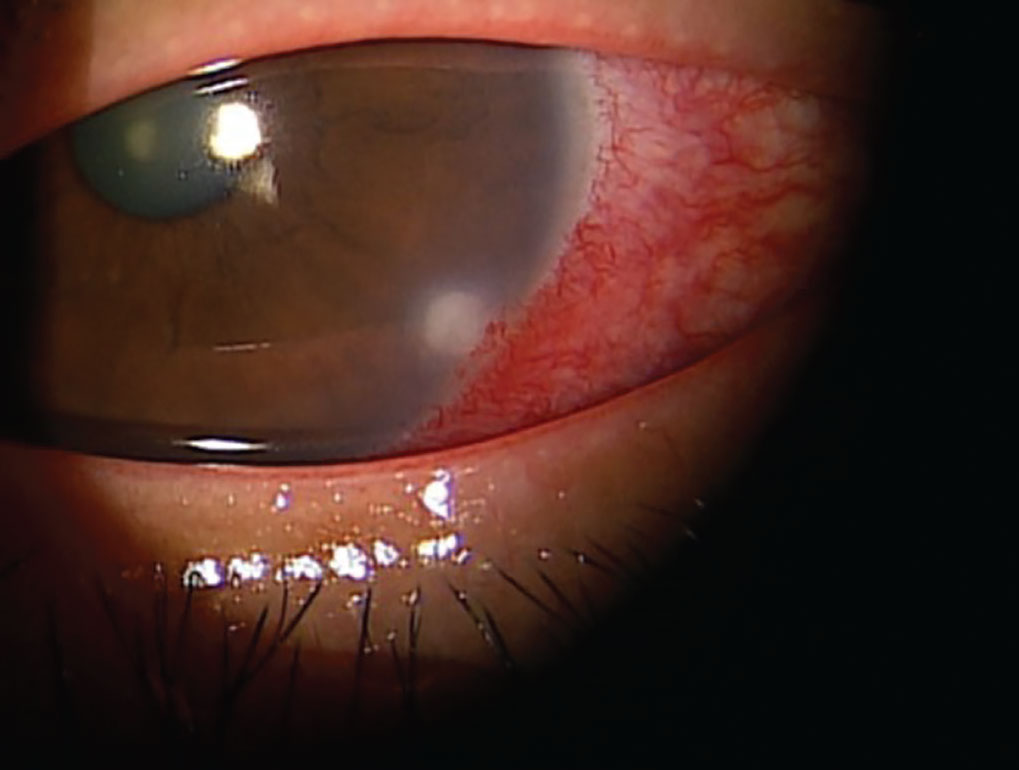 |
|
The common drug dupilumab, used to treat atopic dermatitis, chronic sinusitis and eczema, can cause limbal infiltrates. Click image to enlarge. |
Peripheral corneal infiltrates are also associated with another biologic, adalimumab, which is used to treat a variety of autoimmune conditions, including Crohn’s disease, irritable bowel disease and rheumatoid arthritis.17 Unlike dupilumab-related infiltrates, these have not been shown to progress to ulceration. Since these are less severe, they can typically be treated with topical corticosteroids without discontinuing medication.
Pharmaceuticals can enter the corneal stroma through the aqueous humor, limbal vasculature and tear film.1 Several drugs can cause deposits in the corneal stroma, including chlorpromazine, gold, rifabutin, indomethacin and tyrosine kinase inhibitors, such as vandetanib. Stromal deposits may vary in appearance and can be refractile, pigmented or crystalline.2
Clofazimine is used in combination with other medications to treat lepromatous leprosy, a form of Hansen’s disease. It is also effective against dapsone-resistant lepromatous leprosy and cases of lepromatous leprosy complicated by erythema nodosum leprosum. Clofazimine causes crystalline deposits in the conjunctiva and fine, brownish lines in the anterior corneal stroma, unlike chloroquine keratopathy, which typically resolve upon discontinuation of treatment.18 A pigmentation rate of 53% was reported in patients treated for six to 24 months.19 A different study described polychromatic crystalline deposits accompanied by red-to-brown staining of the bulbar conjunctiva and peripheral cornea; these do not typically impair vision.20
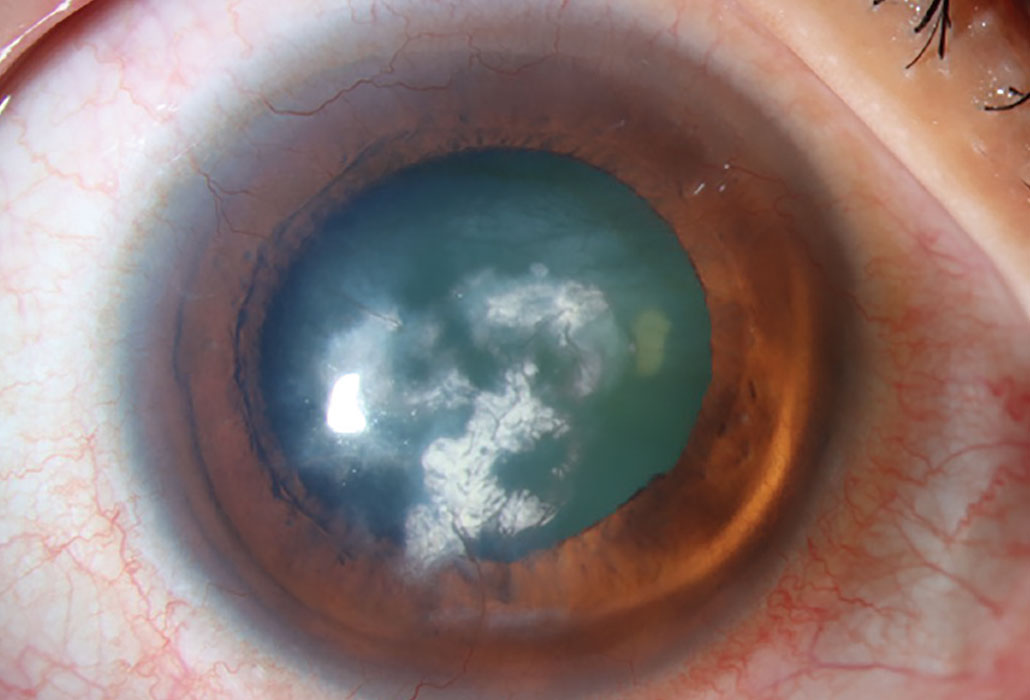
Antipsychotic phenothiazines, particularly chlorpromazine, cause a dose-related, light-induced deposition in the corneal stroma. Phenothiazines are a class of nitrogen- and sulfur-containing heterocyclic compounds classified as first-generation typical antipsychotic medications. They are primarily used to treat schizophrenia, bipolar disorder, control nausea and vomiting and to manage other psychotic disorders characterized by delusional manifestations. With this class of drugs, corneal deposits appear as brown opacities in the posterior stroma, Descemet’s membrane and endothelium.21 The anterior stroma is typically not involved unless high doses are administered. As well as potential opacities, reduced visual acuity may occur with these medications. Chlorpromazine is a phenothiazine antipsychotic that can cause skin and ocular pigmentation when used in high doses over an extended period. It is known to affect the eyelids, conjunctiva, cornea, crystalline lens and, in rare cases, the retina.1
Isotretinoin is used to treat severe nodular acne that is irresponsive to other treatments. It works by reducing excess oil production and altering how skin cells grow and shed, preventing clogged pores and acne formation. Additionally, it helps reduce redness and swelling associated with acne. Corneal opacities are present in the superficial stroma in 5.1% of individuals who take isotretinoin.22 These changes do not affect visual acuity and generally resolve within two to 10 months after drug discontinuation, although some deposits may persist longer in certain individuals. Isotretinoin may also cause epithelial thickening and stromal thinning.23
Crystalline deposits may occur with exogenous immunoglobulin administration. Gold salts, historically used to treat rheumatoid arthritis and though less common today, are administered systemically and can accumulate in the posterior stroma; this is known as ocular chrysiasis.24 Deposits characteristically present following a cumulative dose exceeding 100mg, which is equivalent to seven months of treatment.25 Confocal microscopy has revealed gold salt deposits in the corneal stroma, with every corneal layer containing highly reflective deposits. The largest amount of deposits was located in the anterior stroma in a patient on gold salt treatment for 32 years.26 Additionally, epithelial deposits have been described in 87% of patients who received gold treatment.1 Typically, gold deposits are asymptomatic and do not impair vision, thus treatment discontinuation is not indicated.25
Tyrosine kinase inhibitor medications, such as vandetanib, are used to treat leukemia, medullary thyroid cancer and non-small-cell lung cancer. This type of medication can cause stromal deposition and vortex keratopathy, with symptoms of stromal deposition including blurred vision and tearing.27 Reduced vision occurs with anterior stromal involvement.
Although endothelial deposits are the least common corneal finding, chlorpromazine and rifabutin have been shown to cause deposition in the corneal endothelium.1 Chlorpromazine causes granular whitish-gray opacities that are diffusely distributed across the entire corneal endothelium.28 Rifabutin deposits are refractile and stellate, initially affecting the peripheral cornea before extending centrally. These deposits are a golden hue and can persist despite drug cessation.2 Retroillumination is a helpful tool to diagnose endothelial deposits.1 Most individuals with endothelial deposits are asymptomatic; however, deposits may persist after drug cessation and are occasionally accompanied by retinal dysfunction.
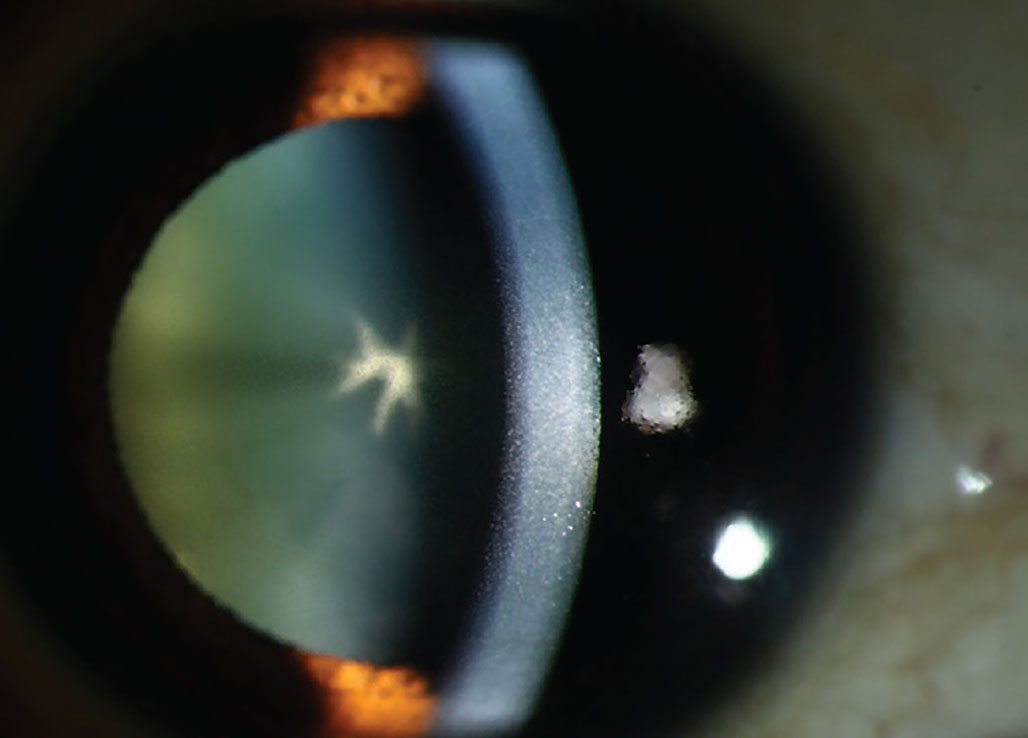 |
|
Chlorpromazine cataract and endothelial deposits. This drug causes deposition in the corneal stroma appearing as brown opacities. Photo: Mile Brujic, OD. Click image to enlarge. |
Some medications that produce corneal deposits can also cause other ocular adverse events, such as retinopathy or optic neuropathy. These medications include amiodarone, tamoxifen, antimalarials and chlorpromazine. The level of corneal findings associated with these medications does not correlate with the risk of optic neuropathy and retinopathy. It is recommended to evaluate and monitor optic nerve and retinal structure and function with retinal imaging, automated visual fields and OCT.1 A dose modification, reduction or change to a different drug must be considered if new visual symptoms are present to avoid visually significant adverse events.
Takeaways
Drug-induced iatrogenic deposits can cause visible opacities that typically affect specific layers or regions of the cornea and may be asymptomatic. Careful examination and a detailed history of all current and historic medications is essential. Detailed examination to determine the layer(s) of the cornea is helpful, since some pharmaceutical agents may affect only a single layer, while others are capable of affecting multiple layers. At times, the findings are incidental only and do not cause symptoms. Alternatively, drug discontinuation may be required due to adverse symptoms. Medication dosage modification or cessation may be beneficial to reduce symptoms and must be evaluated against the systemic risks of the underlying disease. When performing an eye exam, evaluate the cornea and investigate the possible causes of corneal findings. If needed, collaborate with other specialists to provide optimal care.
While managing drug-induced deposits in the cornea is often limited to observation and conservative management, various treatments have been proposed. Topical lubricants or corticosteroids may improve comfort of symptoms. Additionally, autologous serum drops, prescription topical medications, amniotic membranes and scleral lenses may be used based on the clinical findings.
Dr. Barnett, FAAO, FSLS, FBCLA, is a world-renowned key opinion leader recognized for her expertise in specialty and scleral lenses, dry eye disease, keratoconus and presbyopia. She has authored more than 300 articles and books, delivered over 450 lectures, is a board member and founding member of Intrepid Eye Society and serves on several industry boards. Her work has been featured in medical journals, professional magazines, as well as consumer publications such as Bloomberg, Prevention Magazine and Good Housekeeping. Dr. Barnett is most proud of being awarded the Scleral Lens Practitioner of the Year from the Scleral Lens Education Society, the inaugural Theia Award for Excellence for Mentoring by Women In Optometry and was granted the Most Influential Women in Optical from Vision Monday. Dr. Barnett is the Director of Optometry at the University of California, Davis. Her full list of disclosures can be found here.
Dr. Bailey received her Doctor of Optometry degree at Southern College of Optometry. She is currently a cornea and contact lens resident at NSU Oklahoma College of Optometry. She has no financial disclosures.
1. Sahyoun JY, Sabeti S, Robert MC. Drug-induced corneal deposits: an up-to-date review. BMJ Open Ophthalmol. 2022;7(1):e000943. 2. Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004;15(6):541-8. 3. Raizman MB, Hamrah P, Holland EJ, et al. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017;62(3):286-301. 4. Lin JB, Harris JM, Baldwin G, Goss D, Margeta MA. Ocular effects of Rho kinase (ROCK) inhibition: a systematic review. Eye (Lond). September 16, 2024. [Epub ahead of print]. 5. Yuasa T, Takenaka T, Higuchi K, et al. Fabry disease. J Echocardiogr. 2017;15(4):151-7. 6. Nguyen TT, Gin T, Nicholls K, et al. Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Exp Ophthalmol. 2005;33(2):164-8. 7. Mastropasqua L, Nubile M, Lanzini M, et al. Corneal and conjunctival manifestations in Fabry disease: in vivo confocal microscopy study. Am J Ophthalmol. 2006;141(4):709-18. 8. Sodi A, Ioannidis A, Pitz S. Ophthalmological manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: Oxford PharmaGenesis; 2006. Chapter 26. 9. Fitt A, Dayan M, Gillie RF. Vortex keratopathy associated with ibuprofen therapy. Eye (Lond). 1996:10(Pt 1):145-6. 10. Szmyd L, Perry HD. Keratopathy associated with the use of naproxen. Am J Ophthalmol. 1985;99(5):598. 11. Lonial S, Nooka AK, Thulasi P, et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM). Blood Cancer J. 2021;11(5):103. 12. Aschauer J, Donner R, Lammer J, et al. Corneal toxicity associated with belantamab mafodotin is not restricted to the epithelium: neuropathy studied with confocal microscopy. Am J Ophthalmol. 2022;242:116-24. 13. Corbelli E, Miserocchi E, Marchese A, et al. Ocular toxicity of mirvetuximab. Cornea. 2019;38(2):229-32. 14. Important safety information. Pfizer Inc. and Genmab A/S. www.tivdakhcp.com. Accessed October 22, 2024. 15. Tauber J, Ritterband DC, Kang JJ. Corneal complications related to dupilumab use. Eye Contact Lens. 2024;50(6):270-3. 16. Phylactou M, Jabbour S, Ahmad S, Vasquez-Perez A. Corneal perforation in patients under treatment with dupilumab for atopic dermatitis. Cornea. 2022;41(8):981-5. 17. Matet A, Daruich A, Beydoun T, Cosnes J, Bourges JL. Systemic adalimumab induces peripheral corneal infiltrates: a case report. BMC Ophthalmol. 2015;15:57. 18. Font RL, Sobol W, Matoba A. Polychromatic corneal and conjunctival crystals secondary to clofazimine therapy in a leper. Ophthalmology. 1989;96(3):311-5. 19. Wålinder PE, Gip L, Stempa M. Corneal changes in patients treated with clofazimine. Br J Ophthalmol. 1976;60(7):526-8. 20. Barot RK, Viswanath V, Pattiwar MS, Torsekar RG. Crystalline deposition in the cornea and conjunctiva secondary to long-term clofazimine therapy in a leprosy patient. Indian J Ophthalmol. 2011;59(4):328-9. 21. Bernstein HN. Some iatrogenic ocular diseases from systemically administered drugs. Int Ophthalmol Clin. 1970;10(3):553-619. 22. Fraunfelder FT, LaBraico JM, Meyer SM. Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol. 1985;100(4):534-7. 23. Ozyol P, Ozyol E, Yildirim FE. Remodeling of the cornea with isotretinoin treatment. Eye Contact Lens. 2021;47(6):366-77. 24. Sorkin S, Wah N. A review of medications that can affect the cornea. Modern Optometry. modernod.com/articles/2024-may-june/a-review-of-medications-that-can-affect-the-cornea. May/June 2024. 25. Bron AJ, McLendon BF, Camp AV. Epithelial deposition of gold in the cornea in patients receiving systemic therapy. Am J Ophthalmol. 1979;88(3 Pt 1):354-60. 26. Paladini I, Menchini U, Mencucci R. Corneal chrysiasis: in vivo confocal microscopy analysis. Eur J Ophthalmol. 2010;20(4):776-9. 27. Pandit RJ, Innes WA, Strong NP, James RA. Loss of vision? Clear as crystal! Lancet. 2010;375(9714):610. 28. Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990;25(5):249-51. |

