Seeing Double: The Urgent and Chronic Causes of Diplopia
Double vision can originate from many conditions. Learn to recognize when this presentation is an emergency and when it's indicative of a long-term issue.
Goal Statement:
Double vision can occur from many serious conditions involving the neuromuscular anatomy and ocular media. This article will review the chronic conditions (e.g., myasthenia gravis, Graves' disease, multiple sclerosis, diabetes) as well as urgent presentations (e.g., stroke, aneurysm, tumor, giant cell arteritis) that may cause it.
Faculty/Editorial Board:
Jim Williamson, OD
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID is 44941-NO. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Dr. Williamson has no relevant financial relationships to disclose.
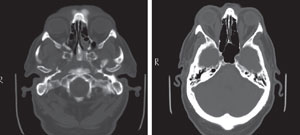 CT scan of the same patient before and after the diagnosis of Graves' disease. Note the markedly enlarged medial recti. |
Optometrists are no strangers to double vision, but when a patient presents with it, ensuring that we correctly identify the cause can be a daunting task. While diplopia can present for numerous reasons, the first thing we need to do is either recognize or rule out urgent conditions, and then (in the latter case) explore the possibility that it may stem from a chronic condition. This article will focus on recognizing the chronic and urgent medical causes of diplopia. These will be loosely grouped by systemic disease, neurological etiologies and eye or orbital conditions.
First, we'll review oculomotor control, as the diagnosis may involve systemic or neurological factors with potentially serious consequences.
Oculomotor Control
Two main pathways are involved in oculomotor function: the supranuclear and infranuclear pathways. The supranuclear route controls pursuits, saccades, vergences and horizontal and vertical gaze. As these are all symmetrical ocular movements, lesions here generally do not prompt diplopic complaints, though exceptions exist which will be discussed later.
The infranuclear pathway begins in each cranial nerve nucleus and proceeds to innervate the respective extraocular muscles (EOMs), as follows:
The oculomotor nerve, CN III, emerges from the anterior aspect of the midbrain. From there, it enters the subarachnoid space in the interpeduncular cistern between the posterior cerebral and superior cerebellar arteries and then runs lateral to the posterior communicating artery (PCA).1,2 After penetrating the arachnoid and internal layer of the dura, it moves into the cavernous sinus, where it lies superior to the trochlear nerve and lateral to the internal carotid artery. The nerve then splits into a smaller superior and larger inferior division, which enter the orbit through the superior orbital fissure.1,2 The superior division supplies the superior rectus and the levator palpebrae. The inferior division further divides into three branches to innervate the inferior oblique and the medial and inferior recti.3 Additionally, the longest inferior oblique branch has parasympathetic fibers that synapse in the ciliary ganglion. The postganglionic fibers pass through the short ciliary nerves to the sphincter pupillae and ciliary muscles.1,2
The trochlear nerve, CN IV, is the only cranial nerve to emerge from the posterior aspect of the midbrain.3 As it supplies just one muscle—the superior oblique—the trochlear is the smallest of all cranial nerves and yet has the longest intracranial course.1,2 It moves laterally and forward in the sub-arachnoid space around the cerebral peduncle and enters the lateral wall of the cavernous sinus inferior to the oculomotor nerve. Continuing through the superior orbital fissure above the fibrous ring, it arrives at the superior oblique muscle as a series of small branches.1,2
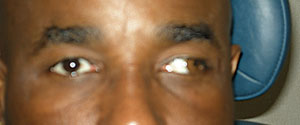 Due to its often transient nature and variability, treating pathologic diplopia can be challenging. Often, treatment consists of use of Fresnel prisms or occlusion. Younger patients or those concerned with cosmesis can further limit the choices. Such was the case for this MS patient with a right INO. The solution entailed fitting the patient in a tinted soft contact lens with an opaque pupil. |
The abducens nerve, CN VI, leaves the ventral aspect of the brainstem between the border of the pons and medulla oblongata. It ascends through the subarachnoid space along the clivus as it courses anterolaterally through the pontine cistern.1,2 Following a sharp bend over the petrous part of the temporal bone (which makes it vulnerable to injury and increased intracranial pressure), it passes through the cavernous sinus where it first lies lateral and then inferolateral to the internal carotid artery.3 Like CN III and IV, the superior orbital fissure provides orbital access to the abducens nerve, where it enters the medial side of the lateral rectus muscle.1,2
Systemic Diseases
Eye care practitioners must evaluate whether the onset of diplopia is a sign of a larger, systemic disease. Patients presenting with diplopia may or may not be aware of systemic conditions behind their visual symptom. These can include life-altering diagnoses such as Alzheimer's, Parkinson's or giant cell arteritis. Here are ways optometrists can learn to recognize the signs associated with these diplopia-causing systemic diseases:
• Myasthenia gravis. Optometrists are often the first to encounter myasthenia gravis (MG), as many of these patients have ocular sequelae such as ptosis and diplopia.4 In MG, acetylcholine receptor (AChR) antibodies block effective transmission of the chemical to muscle synapses, resulting in weakness. In addition to ophthalmic involvement, MG may also lead to arm and leg fatigue, difficulty chewing and swallowing, speech problems and, in severe cases, breathing impairment.
The key to distinguishing this from other neurological disorders lies with the fluctuation in symptoms, which are typically worse as the day progresses. Remember that ptotic patients may not complain of diplopia, due to occlusion. Extra-ocular muscles should be examined with the lid retracted. Statin use has been associated with MG and, though rare, should be considered, given the widespread use of this drug class.5
Three quick and simple in-office tests can help confirm the suspicion of MG. First, ask the patient to sustain upgaze while you look for superior rectus or levator fatigue. Second, perform the highly specific ice pack test by placing ice on the eyelid for two minutes and evaluating for ptosis improvement.6 Third, evaluate for orbicularis oculi weakness by attempting to open the patient's eyelids after forceful closure. For bloodwork, order the AChR-antibody tests (binding, blocking and modulating), but keep in mind that this can be negative in 45% to 65% of patients with ocular symptoms only (i.e., ocular myasthenia gravis).7
• Parkinson's disease. Among the neurological conditions that can cause diplopia, only Alzheimer's has a higher prevalence than Parkinson's disease, so optometrists can expect to see many of these patients.8 Degenerating cells within the substantia nigra result in a biochemical loss of the neurotransmitter dopamine, which in turn may affect oculomotor control. Saccades, pursuits and vergences can all be involved. Dopaminergic treatment, though effective in providing some improvement in gross motor skills, has little effect on oculomotor deficits, so even well-controlled Parkinson's disease patients can report visual changes.9 Remember when assessing EOMs in elderly patients that some restriction may occur even without a pathological cause. This typically involves upgaze and could be due to changes in orbital tissue.10 Though optometrists don't treat Parkinson's disease, some authors suggest obtaining an OCT scan to measure macular ganglion cell/inner plexiform layer thickness as a way to monitor its progression.11
• Microvascular disease. In 2013 researchers reviewed 109 patients with isolated CN III, IV and VI palsies and identified microvascular ischemia as the cause in nearly 85% of cases.12,13 At risk are those with diabetes, hypertension or hyperlipidemia, as well as patients over 60.
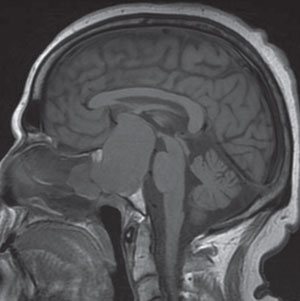 A 63-year-old patient presented with a complaint of decreased visual acuity OU. Confrontational visual fields picked up a bitemporal hemianopsia. Subsequent MRI showed a massive 5.7cm x 6.0cm x 8.8cm pituitary macroadenoma. |
Because of this, consensus opinion varies regarding the need for costly neuroimaging of these infranuclear incidents.12,14 The decision to delay should coincide with the clinician's ability to obtain a thorough history, uncover systemic symptoms and locate relevant ophthalmic findings. Experts agree, however, on the need for an immediate radiological work-up in the following scenarios:14
- patients younger than 50
- history of cancer
- evidence of combined cranial neuropathies
- incomplete or pupil-involving CN III palsies
Regarding the latter, up to 20% of ischemic CN III palsies have anisocoria, but often less than 2mm.
Features of aberrant regeneration of CN III indicate a non-microvascular etiology necessitating immediate work-up.
If the CN palsy is microvascular in etiology, it should show significant resolution in three months (the 90-day rule). If neuroimaging is not done, the patient needs to be monitored closely over the first several months. If the magnitude of the deviation worsens, or does not improve as expected with microvascular disease, obtain prompt neuro-imaging.
• Orbital inflammatory disease. Thyroid eye disease can be caused by hyperthyroidism, Hashimoto's thyroiditis and primary hypothyroidism. Of these, Graves' disease is the most common. In this autoimmune disorder, antibodies bind to the thyroid surface and stimulate the overproduction of thyroid hormones, which leads to hyperthyroidism. Approximately half of patients will develop thyroid-associated orbitopathy (TAO)—a type of orbital inflammatory disease.15 Extraocular muscle inflammation or fibrosis limits eye movement and produces diplopia. The inferior rectus is often affected first, followed by the medial, superior and lateral recti.16
Smoking is a well-known, though not completely understood, risk factor for TAO. Some say it activates the pathways associated with adipogenesis and inflammation.17 Regardless, Graves' disease patients should be advised to quit.
Several recent studies suggest a relationship between TAO and elevated immunoglobulin G4 (IgG4).18,19 They advocate screening for these levels to determine the likelihood of developing TAO.19 Patients with increased IgG4 tended to be older, responded well to anti-thyroid medications, and were more prone to hypothyroid after drug treatment.18
Other systemic inflammatory conditions associated with orbital inflammatory disease include sarcoidosis and granulomatosis with polyangiitis (GPA), formerly known as Wegener's. Non-caseating granulomas characterize sarcoidosis, along with hilar lymphadenopathy seen on chest X-ray and elevated serums levels of angiotension converting enzyme (ACE). GPA is a disease of small- and medium-sized blood vessels, with patients having abnormal levels of cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA). Additionally, infection and tumor must be considered in orbital inflammatory disease cases.
• Giant cell arteritis. The most common systemic vasculitis, giant cell arteritis is also the most feared from an ophthalmic standpoint due to the potential for permanent vision loss.20 While the incidence of diplopia with giant cell arteritis is small (between 2-15%),21,22 it deserves mentioning, especially if it occurs with other symptoms such as headache, neck pain, fever, malaise, weight loss, jaw claudication and temporal artery tenderness. Also, diplopia usually precedes visual loss and may be an early indication of giant cell arteritis.21 If suspected, immediately order an erythrocyte sedimentation rate and C-reactive protein. A positive temporal artery biopsy confirms the diagnosis,22 but a negative result does not exclude it.21 Prompt diagnosis and treatment with high-dose systemic corticosteroids are keys to a more favorable outcome.
Table 1. Questions to Ask Patients with Diplopia
|
Neurological Etiologies
When double vision begins in the brain, the cause can range from a mild injury to serious conditions such as a tumor or aneurysm. To narrow down the possibilities, get a proper history and understand which patients are at-risk with the following guide:
• Traumatic brain injury. Types and severities of TBI range broadly from a mild concussion to those involving loss of consciousness, post-traumatic amnesia, disorientation or other neurological disorders.23 Dysfunctions of oculomotor control may be seen with all classi fications. Symptoms include blurred vision, difficulty following targets, reading problems and diplopia.24
It is important to note that some patients experience a delayed onset of symptoms—therefore, a remote history of head trauma could prove significant. One of the most common issues in mild TBI is convergence insufficiency.
• Supranuclear lesions. As noted previously, the supranuclear pathway controls symmetrical eye movements; thus, lesions here should not cause diplopia. The two main execeptions are internuclear ophthalmoplegia (INO) and skew deviation. INO stems from a lesion in the highly myelinated medial longitudinal fasciculus, which connects the abducens nuclei in the pons to the oculomotor nuclei in the midbrain.25
Patients with INO are unable to adduct and may have a nystagmus when abducting the fellow eye.
A bilateral INO presentation or occurrence in a young individual frequently indicates multiple sclerosis (MS), while vascular ischemia accounts for the majority of unilateral or elderly INO cases.26 Convergence may be affected in some patients, depending on the location of the lesion. The center for convergence is in the midbrain. So, midbrain lesions affect convergence while pontine lesions spare it.
Skew deviation is a vertical misalignment that does not localize to any one particular cranial nerve or cyclovertical muscle (i.e., superior and inferior recti or obliques). Skew deviation most commonly results from faulty prenuclear vestibular input to the ocular motor nuceli or damage to the MLF in the brain-stem.27,28 It is most commonly associated with cerebellum, brainstem or vestibular lesions. When skew deviation is accompanied by ocular torsion and head tilt, it is called the ocular tilt reaction.28
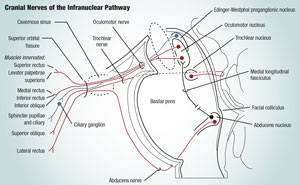 The oculomotor nerve (CN III), the trochlear nerve (CN IV) and the abducens nerve (CN VI) are key to the infranuclear pathway, one of the two main pathways involved in oculor motor controls. Such functions may be affected by systemic or neurological diseases that cause diplopia. These diseases can have potentially serious side consequences. |
• Aneurysm. A common location for intracranial aneurysms is the junction of the internal carotid artery and the posterior communicating artery.29 It should be no surprise, then, that an aneurysm in this area puts the oculomotor nerve at risk, since it runs lateral to the PCA. Also, since pupillomotor fibers run on the surface of CN III, a compressive aneurysm would likely produce pupillary involvement, which may be the first feature. An aneurysm should be ruled out in any CN III palsy with associated pain or full/ partial pupillary involvement, especially in patients less than age 50 or without vasculopathic risk factors.30
Standard of care in these patients dictates emergent referral with immediate imaging. While the gold standard for aneurysm detection is cerebral catheter angiography, com puterized tomographic angiography (CTA) is preferred.31 Should a contraindication exist for contrast dye (e.g., pregnancy, allergy, impaired renal function), a magnetic resonance angiography (MRA) would be performed. CTA takes only a few minutes, with the bulk of time spent on image post-processing. It outperforms MRA in detecting aneurysms <5mm by an impressive 94% to 54% margin, making it an ideal test, given the typical size of a third-nerve palsy aneurysm.31
• Cavernous sinus. Since the cavernous sinus plays host to all the oculomotor nerves, any primary or extending secondary pathology affecting this structure may produce diplopic complaints. Two processes that originate here are carotid-cavernous fistula (CCF) and Tolosa-Hunt syndrome (THS).
A CCF is an abnormal communication that allows blood to flow from the carotid artery into the cavernous sinus. The broad classifications of CCF are direct vs. indirect.
A direct CCF arises from a traumatic or aneurysmal rupture etiology, progresses rapidly and requires urgent treatment.32 In contrast, an indirect CCF tends to be more insidious in onset, leading to a delay in diagnosis due to its chronic nature.32 Clinicians should add indirect CCF to their differentials when treating conjunctival injection, as this is a common ocular sequelae.
THS is an idiopathic, nonspecific inflammation of the cavernous sinus, causing painful ophthal-moplegia. These changes should be evident on neuroimaging, but not always. The term “benign THS” applies when this occurs.33
• Tumor. A pituitary adenoma is a familiar entity in the eye care field due to its potential ophthalmic manifestations. Two types exist, differentiated by size: microadenoma (<10mm) and macroadenoma (>10mm). Only the latter produces ocular findings, due to compression of the optic chiasm and resulting visual field defects. Further expansion of the tumor into the cavernous sinus may elicit a CN III, IV or VI palsy. Of those, CN III and the levator palpebrae superioris are the most affected nerve and muscle, respectively.34
While a macroadenoma itself is not typically an urgent situation, acute hemorrhage or ischemia within the pituitary tumor produces the emergent and possible life-threatening condition pituitary apoplexy (PA). Headache, nausea and impaired mental status join decreased visual acuity and ophthalmoplegia as common presenting symptoms, which develop from hours to days, or longer.35
Eye and Orbital Conditions
When neither a systemic disease nor a neurological condition can explain diplopia, the problem may be limited to the eye itself. Here are several possible eye and orbital conditions that can lead to double vision:
• Herpes zoster ophthalmicus. Ocular complications arise when the latent varicella zoster virus (VZV) invades the ophthalmic division of CN V during a shingles outbreak, resulting in herpes zoster ophthalmicus (HZO). Cranial nerve palsies occur in up to 30% of HZO patients.36 CN III is affected the most, followed by CN VI and CN IV.37 Perform a careful EOM evaluation, as over 25% remain asymptomatic with diplopia only being noticed in extreme gaze.38
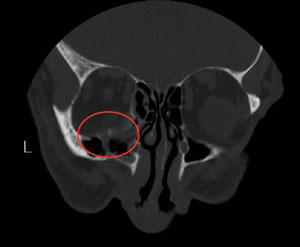 CT scan showing orbital floor blow-out fracture. |
A gradual decline in immunity to varicella zoster virus has led to increased rates of herpes zoster (HZ) and postherpetic neuralgia (PHN).39 Some suggest the cause is decreased exposure to VZV stemming from the 1996 recommendation for immunization in children. Repeated exposure, they say, boosts a person's cell-mediated immunity against the virus. However, one of several studies does not support that conclusion and notes accelerated rates even before the vaccine's introduction.40
The Advisory Committee on Immunization Practices (ACIP) and Centers for Disease Control (CDC) recommend the 2006 FDA approved zoster vaccine Zostavax for patients age 60 or older. The Shingles Prevention Study showed markedly reduced morbidity from HZ and PHN and an over 50% reduction in HZ incidence.41 Screen younger patients with HZ for an underlying immunocompromising etiology, such as HIV.
• Idiopathic orbital inflammatory disease. After ruling out all potential causes of OID, the clinician is left with the diagnosis of exclusion—idiopathic orbital inflammatory disease (IOID). Oral steroids remain the definitive treatment for IOID, with the expected course being rapid improvement on these agents. It is of note that lymphoma can mimic IOID, and can initially respond to steroid treatment, delaying its diagnosis and proper treatment.
Immunosuppressive drugs can also be used. Some cases are associated with abnormal IgG4 levels, where patients initially respond to glucocorticoids but relapse during taper.42 Several have reported successful treatment using rituximab—a monoclonal antibody also used in GPA.42,43
Table 2. Tests for Diplopia
|
• Orbital blow-out fractures. Ocular trauma renders the small and thin orbital bones susceptible to damage, with orbital floor fractures being the most common.44 Since the infraorbital nerve runs through this area, patients often cite hypoesthesia along its distribution (i.e., cheek and upper lip). The incidence of diplopia, usually involving upgaze, varies; it has been reported to be from 20% to 45%.44,45 The diplopia occurs due to extraocular muscle edema, nerve palsy or an entrapped muscle within the fracture. A longstanding entrapment often leads to fibrosis.
Though typically not emergencies, urgent surgery should occur if the oculocardiac reflex can be stimulated, a muscle is entrapped or the optic nerve is affected.46 Non-urgent repair (within two weeks) depends on diplopic complaints and the extent of patient distress from the ensuing enophthalmos.
• Superior orbital fissure syndrome. The superior orbital fissure provides orbital access to not only CN III, IV and VI as previously discussed but also the frontal, lacrimal and nasociliary branches of the ophthalmic nerve.1,2 An infiltrative, inflammatory, ischemic or traumatic event affecting this 3mm x 22mm area is called superior orbital fissure syndrome (SOFS).47 With all the nerves traversing through this location, SOFS results in ophthalmoplegia, forehead and upper eyelid hypoesthesia, ptosis and pupillary mydriasis.
Loss of extraocular muscle tone—which usually exerts a retracting force on the globe—leads to proptosis.47 Vision loss indicates optic nerve involvement, and when this occurs the diagnosis changes from SOFS to orbital apex syndrome (OAS). The latter is a more urgent condition that may require early intervention such as high-dose corticosteroids or surgical decompression of the optic canal, depending on the etiology.48
Conclusion
Though neurologic imaging is important when dealing with diplopia, it is only helpful when one knows the anatomic location upon which to focus attention. Nothing can replace a thorough history and clinical exam. Using these, optometrists can try to localize the diplopia and determine if it is likely a process of systemic or neurologic disease, or an orbital condition. This will help establish the urgency of the situation and the need for additional imaging, treatment or further referral.
References
- Snell R, Lemp M. The Orbital Nerves. In: Clinical Anatomy of the Eye. Cambridge: Blackwell;1989:267-76.
- Moore, Keith L. Clinically Oriented Anatomy, 3rd ed. Baltimore: Wil-liams & Wilkins; 1992:859-65.
- Koskas P, Héran F. Towards understanding ocular motility: III, IV and VI. Diagn Interv Imaging. 2013;94(10):1017-31.
- Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32(3):215-26.
- Gale J, Danesh-Meyer H. Statins can induce myasthenia gravis. J Clin Neurosci. 2014;21(2):195-7.
- Rajasekharan C, Anishkumar V, Suresh MK. Ice pack test: is it obsolete? BMJ Case Rep. 2011;2011:1-2.
- Lee J, Koh K, Kim U. The anti-acetylcholine receptor antibody test in suspected ocular myasthenia gravis. J Ophthalmol. doi:10.1155/2014/689792
- Goetz C, Pal G. Initial management of Parkinson's disease. BMJ. 2014;349:g6258.
- Pinkhardt EH, Jürgens R, Lulé D, et al. Eye movement impairments in Parkinson's disease: possible role of extradopaminergic mechanisms. BMC Neurol. 2012;12(1):5.
- Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2004;45(3):729-38.
- Sari E, Koc R, Yazici A, et al. Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J Neuroophthalmol. 2014;(6). doi: 10.1097/WNO.0000000000000203
- Tamhankar M, Biousse V, Ying G, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. e2013;120(11):2264-9.
- Chou K, Galetta S, Liu G, et al. Acute ocular motor mononeu-ropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci. 2004;219(1–2):35-9.
- Murchison A, Gilbert M, Savino P. Neuroimaging and acute ocular motor mononeuropathies: A prospective study. Arch Ophthalmol. 2011;129(3):301-5.
- Stein J, Childers D, Gupta S, et al. Risk factors for developing thyroid-associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmol. 2014;48105:1-7.
- Kanski, Jack J. Clinical Ophthalmology, 6th ed. Edinburgh: Butterworth-Heinemann; 2007:171-174.
- Planck T, Shahida B, Parikh H, et al. Smoking Induces Overexpres-sion of Immediate Early Genes in Active Graves' Ophthalmopathy. Thyroid. 2014;24(10):1524-1532.
- Takeshima K, Inaba H, Furukawa Y, et al. Elevated Serum Immuno-globulin G4 Levels in Patients with Graves' Disease and Their Clinical Implications. Thyroid. 2013;24(4):736-743.
- Bozkirli E, Bakiner OS, Ersozlu Bozkirli ED, et al. Serum Immuno-globulin G4 levels are elevated in patients with Graves' ophthalmopa-thy. Clin Endocrinol (Oxf). 2014:1-6.
- Gonzalez-Gay M, Garcia-Porrua C, Llorca J, et al. Visual Manifestations of Giant Cell Arteritis. Medicine (Baltimore). 2000;79(5):283-92.
- Rahman W, Rahman F. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol. 2005;50(5):415-28.
- Hayreh S, Podhajsky P, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125(4):509-20.
- Goodrich GL, Martinsen GL, Flyg HM, et al. Development of a mild traumatic brain injury-specific vision screening protocol: a Delphi study. J Rehabil Res Dev. 2013;50(6):757-768.
- Ciuffreda K, Kapoor N, Rutner D, et al. Occurrence of oculomotor dysfunctions in acquired brain injury: A retrospective analysis. Optom -J Am Optom Assoc. 2007;78(4):155-61.
- Wang C, Paling D, Chen L, et al. Axonal conduction in multiple sclerosis: A combined magnetic resonance imaging and electro-physiological study of the medial longitudinal fasciculus. Mult Scler. 2014:1-11.
- Karatas M. Internuclear and supranuclear disorders of eye movements: clinical features and causes. Eur J Neurol. 2009;16(12):1265-77.
- Hernowo A, Eggenberger E. Skew deviation: clinical updates for ophthalmologists. Curr Opin Ophthalmol. 2014;25(6):485-87.
- Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol. 2005;51(2):105-28.
- Chang S, Tsai M, Wei C. Posterior communicating aneurysm with oculomotor nerve palsy: clinical outcome after aneurysm clipping. Turk Neurosurg. 2014;24(2):170-3.
- Eggers SDZ, Bundrick JB, Litin SC. Clinical pearls in neurology. Mayo Clin Proc. 2012;87(3):280-5.
- Chaudhary N, Davagnanam I, Ansari SA, Pandey A, Thompson BG, Gemmete JJ. Imaging of intracranial aneurysms causing isolated third cranial nerve palsy. J Neuroophthalmol. Sep 2009(3)238-44.
- Ellis JA, Goldstein H, Connolly ES, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus. 2012;32(5):E9.
- Hung C-H, Chang K-H, Chu C-C, et al. Painful ophthalmoplegia with normal cranial imaging. BMC Neurol. 2014;14:7.
- Fraser C, Biousse V, Newman N. Visual outcomes after treatment of pituitary adenomas. Neurosurg Clin N Am. 2012;23(4):607-19.
- Bi W, Dunn I, Laws Jr. E. Pituitary apoplexy. Endocrine. 2014:1-7.
- Chaker N, Bouladi M, Chebil A, Jemmeli M, Mghaieth F, El Matri L. Herpes zoster ophthalmicus associated with abducens palsy. Journal of Neurosciences in Rural Practice 2014;5(2):180-2.
- Harthan J, Borgman C. Herpes zoster ophthalmicus-induced oculo-motor nerve palsy. J Optom. 2013;6(1):60-5.
- Marsh R, Dulley B, Kelly V. External ocular motor palsies in ophthalmic zoster: a review. Br J Ophthalmol . 1977;61(11 ):677-82.
- Centers for Disease Control and Prevention. http://www.cdc.gov/ shingles/hcp/clinical-overview.html. Accessed January 5, 2015.
- Hales C, Harpaz R, Joesoef M, Bialek S. Examination of links between herpes zoster incidence and childhood varicella vaccination. Annals of Internal Medicine. 2013:159(11):739-45.
- Oxman M, Levin M, Johnson G, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-84.
- Wallace Z, Khosroshahi A, Jakobiec F, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol. 2012;57(1):26-33.
- Shao E, Karydis A, Gemenetzi M, Taylor S. Successful corticosteroid-sparing effect of rituximab in the treatment of refractory idiopathic orbital inflammatory disease. Case Rep Ophthalmol. 2013;4(3):216-8.
- Bartoli D, Fadda M, Battisti A, et al. Retrospective analysis of 301 patients with orbital floor fracture. J Craniomaxillofac Surg. 2014:1-4.
- Chi M, Ku M, Shin K, Baek S. An analysis of 733 surgically treated blowout fractures. Ophthalmologica. 2010;224(3):167-75.
- Gart M, Gosain A. Evidence-based medicine: orbital floor fractures. Plast Reconstr Surg. 2014;134(6):1345-55.
- Heath H, Wurth B, Penna K. Superior orbital fissure syndrome: A case report. Craniomaxillofac Trauma Reconstr. 2012; 5(2): 115–20.
- Sugamata A. Orbital apex syndrome associated with fractures of the inferomedial orbital wall. Clin Ophthalmol. 2013; 7: 475–78.
