 |
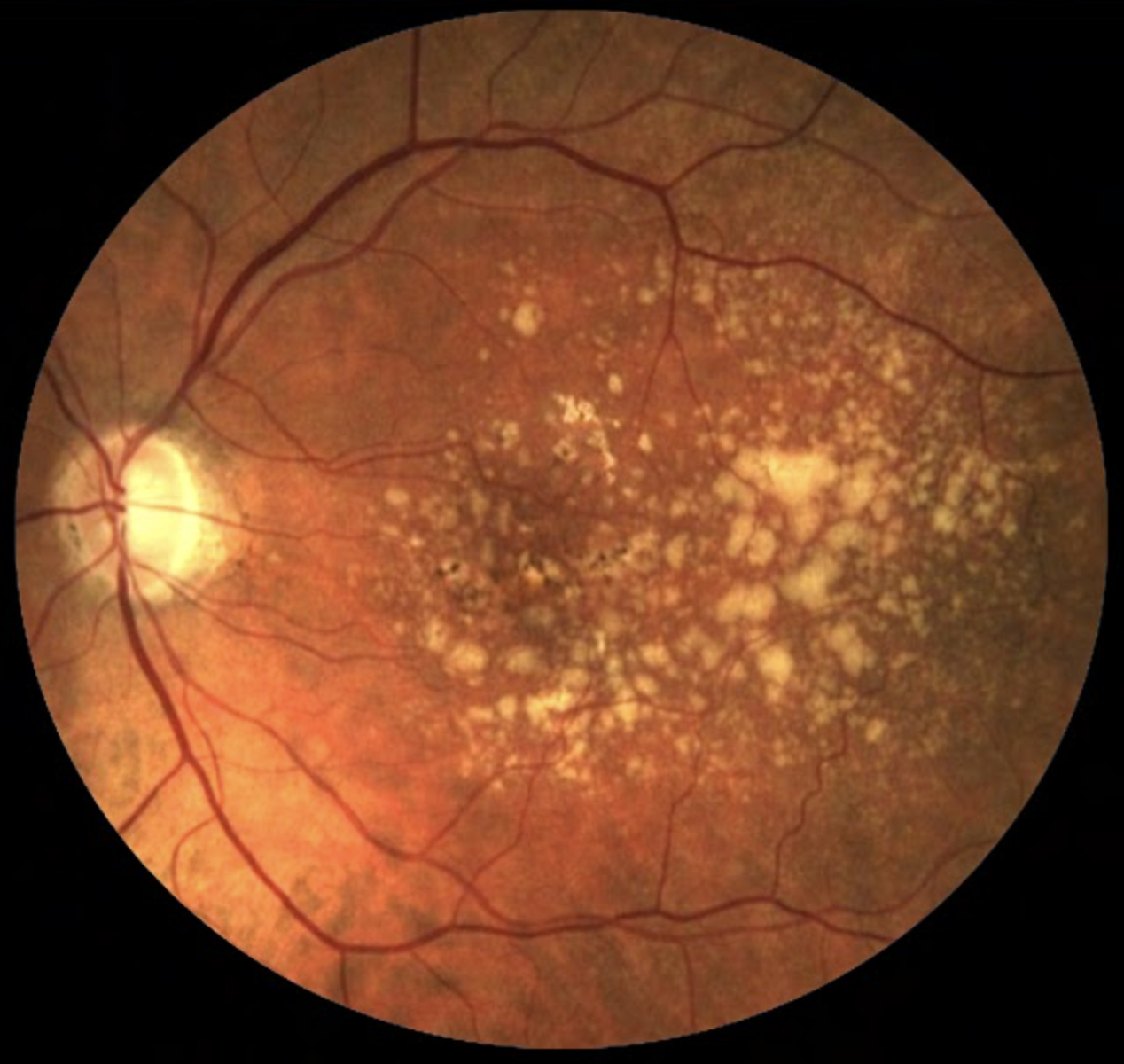
Of the three identified blood metabolites, hypoxanthine may act as a potential free radical generator and serve as an indicator of hypoxic conditions, while 2-furoylglycine is a potential marker of coffee intake, with caffeine metabolism exhibiting potentially perturbed nature in AMD patients. The third agent, 1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine, is less understood in its role with the condition. Photo: NEI. Click image to enlarge. |
Being the leading cause of irreversible blindness worldwide, age-related macular degeneration (AMD) poses significant challenges for early diagnosis and treatment. With such a devastating impact, researchers are looking to decrease the amount of visual impairment associated with disease, which could be achieved through implementing effective early screening programs.
Right now, retinal imaging advancements have allowed for biomarkers to be identified indicating late-stage AMD progression; however, a screening test is still crucial for the general population—with good sensitivity and great specificity—due to the asymptomatic nature of early-stage AMD. No such screening test currently exists for this early stage that meet these performance standards, though, prompting researchers in Shanghai to investigate blood serum metabolites as potential biomarkers.
Included in the cohort from two different centers was a total of 547 people; 167 were healthy controls, 240 were controls who had other eye diseases and 140 were individuals with AMD. These subjects were studied over three phases: discovery phase I, discovery phase II and an external validation phase. In both discovery phases, a machine learning algorithm was built to predict AMD probability, while the external validation phase further confirmed the performance of the biomarker panel identified by the algorithm. Subsequently, the researchers evaluated the performance of the identified biomarker panel in monitoring progression and severity of AMD.
Developed from this process was the three-metabolite panel, which includes hypoxanthine, 2-furoylglycine and 1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine, after running five machine learning models. A random forest model effectively discriminated AMD patients from those in the other two groups with acceptable calibration in both discovery phases, and an independent validation phase confirmed the diagnostic model’s efficacy. An area under the curve of 1.0, indicated perfect correlation, was achieved by the three-biomarker panel to differentiate AMD severity by random forest machine learning, and this was consistent across all phases, indicating it maintained exemplary diagnostic performance in differentiating AMD from other ocular conditions. Even greater, after multiple freeze-thaw cycles of the study samples, biomarker concentrations remained stable.
In the discussion section of their paper, the authors of the study further distinguish that the three identified distinct metabolites indicative of AMD increased progressively from nonprogressive to progressive stages of the disease, suggestive of them for potential as dependable markers for monitoring both progression and severity. What’s more, the three agents held a notable ability in differentiating between varying AMD severities. With both considerations in mind, the authors relay that “these observations provide robust preliminary evidence that the levels of these serum biomarkers could be effectively utilized as innovative markers for early identification, as well as for tracking the advancement and intensity of AMD.”
| Click here for journal source. |
Li S, Qui Y, Li Y, et al. Serum metabolite biomarkers for the early diagnosis and monitoring of age-related macular degeneration. J Adv Res. 2024:S2090-1232(24)00434-X. |

