 |
As optometrists, we are integral to treating both dry eye disease (DED) and glaucoma at the earliest stages, yet our care is too often reactive. Research shows the comorbidity of DED in patients treated topically for ocular hypertension and glaucoma could be as high as 59%.1 We have many strategies in our arsenal to help patients get the glaucoma treatments they need without necessarily compromising their ocular surface health. These include smart medication prescribing and glaucoma surgery, whether it’s traditional options or minimally invasive glaucoma surgery (MIGS).2-4
Glaucoma Med Conundrum
Topical IOP-lowering medications, the mainstay of glaucoma therapy, are a godsend for those who need them. But all five classes of medication used—beta blockers (β-blockers), carbonic anhydrase inhibitors (CAIs), prostaglandin analogs (PGAs), alpha 2-adrenergic agonists and rho-kinase inhibitors—come with adverse reactions, many of which are localized to the ocular surface. These can impact everything from ocular surface health to diagnostic testing and compliance.
Ocular surface. Allergy, toxicity, immuno-inflammatory effects, superficial punctate keratitis, conjunctival inflammation and disruption of the tear film are all among the many side effects of both the glaucoma medications and the compounds used to preserve them.5,6
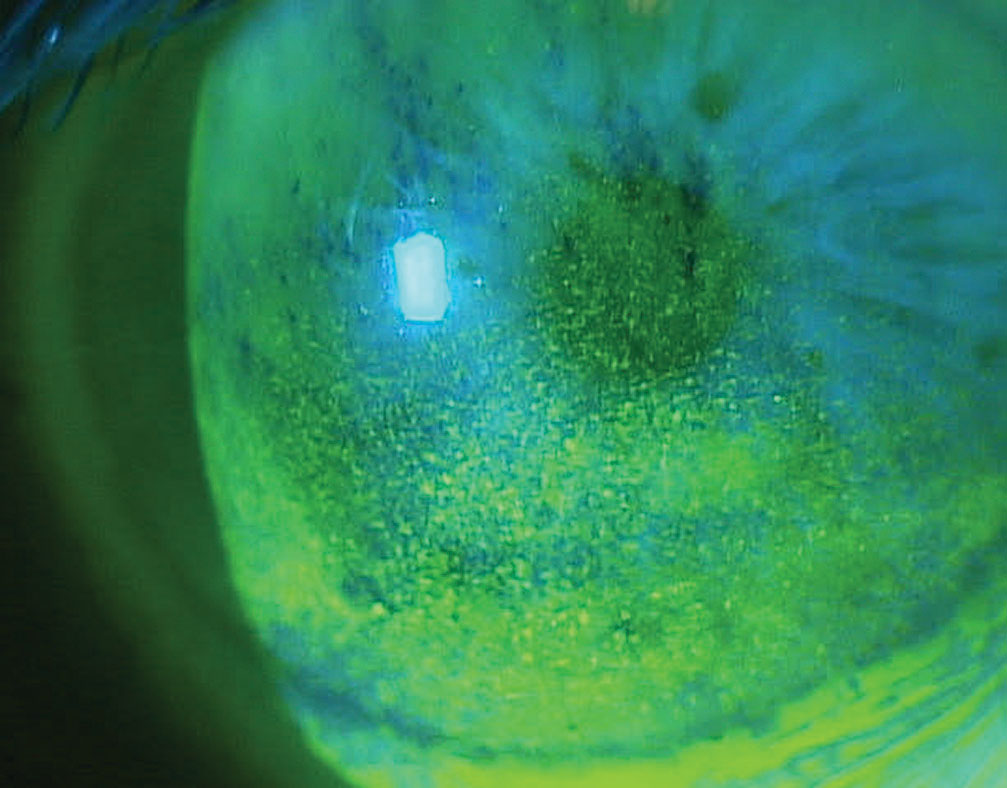 |
| Corneal superficial punctate keratitis in a patient on multiple topical glaucoma medications. Click image to enlarge. |
Preservatives such as benzalkonium chloride (BAK) are classified as quaternary ammonium compounds that act as a detergent and can disrupt the cell membrane, reduce epithelial cell proliferation and decrease corneal epithelial tight junctions.7,8 These detergents disrupt the lipid layer, resulting in a decreased break-up time and the potential for evaporative dry eye issues.9 Although both preserved and preservative-free PGAs cause meibomian gland dysfunction (MGD), preserved forms are more toxic to the meibomian glands, resulting in a greater reduction of mean acinar cell density and area, less homogeneity and significant alterations to secretions.10 All of this means the meibomian glands are more prone atrophy.
One study looking at the effects both β-blockers and PGAs on meibomian gland morphology found a positive correlation between topical treatments and decreased gland structure and function.11
PGAs are pro-inflammatory. Such chronic inflammation not only affects epithelial cell function but can also damage the structure and function of sensitive oil-producing meibomian glands.12 One study found more than 90% of patients on PGAs had MGD.13 PGAs also induce a significant involution of meibomian lipoid activity.14
A recent study found signs of corneal and conjunctival ocular surface disease were evident in 44% of patients treated with PGA therapy.5 Goblet cells on the conjunctiva, the main source of muco-proteins, are essential in preventing conjunctival epithelial disquamation, inflammation and cell death. Numerous studies show a significant loss of goblet cell density with long-term use of preserved glaucoma medications, and PGAs in particular.15-18
The prevalence of blepharitis, MGD and dry eye is more than twice as high after the commencement of topical glaucoma therapy compared with before therapy, and with each additional medication the risk of an adverse event or possible exacerbation of DED multiplies.5,19-21
Long-term topical glaucoma therapy can so drastically disrupt the ocular surface that it can impact future surgical options if the condition continues to progress.22 For example, a disrupted ocular surface results in inaccurate keratometry readings, which could directly affect intraocular lens calculations. It can also lead to scarring that can make shunt and trabeculectomy procedures difficult or less effective.
Diagnostic testing. Tear film disruption can affect the reliability and reproducibility of diagnostic testing such as visual fields.23 One study found treating glaucoma patients’ DED for one week with just artificial tears improved their visual field test duration, mean deviation and the number of depressed points.23
Compliance. Topical glaucoma therapy’s effect on the ocular surface can lead to decreased medication compliance—a known risk factor for disease progression.24 Patients who have problems with their topical glaucoma medication are at higher risk for poor compliance, with typical medication non-adherence ranging between 50% and 60%.5,25
Cut the Drops with SurgeryAlternative therapies such as selective laser trabeculoplasty (SLT) or MIGS may eliminate medication-induced effects, reducing the risk for dry eye symptoms. The largest MIGS trial illustrates the capacity of this approach to reduce or eliminate patients’ dependence on medications. The HORIZON trial compared the efficacy of the Hydrus microstent (Ivantis) plus cataract surgery vs. cataract surgery alone in mild to moderate glaucoma. Two years post-op, 78% of patients with the combination surgery were medication-free, an improvement of 30% over those with cataract surgery alone.4 Studies show MIGS procedures have a lower risk profile than conventional surgery—a fact that is slowly leading to Until recently, surgery was an unappealing option. With filtering surgery, we worried about infection, hypotony, inflammation and bleb-related complications. And although SLT is an excellent treatment option for many, it demonstrates diminished efficacy over time.26 MIGS, on the other hand, offers an entirely different approach and effectively addresses the needs of patients with mild to moderate glaucoma. What’s more, MIGS has a good safety profile and patients recover rapidly.1 |
Smart Prescribing
While keeping IOP under control is a primary aim, practitioners must balance this alongside the practical realities that are inherent with the long-term use of topical therapy.
Preservatives such as BAK are added to topical medications to prevent microbial proliferation after opening; however, they frequently cause or aggravate ocular surface dis- ease in glaucoma.27,28 For glaucoma treatment to be effective, clinicians must minimize the side effects to pro- mote compliance.29
Manufacturers are increasingly developing preservative-free drops and combination therapies aimed at minimizing exposure to preservatives and other irritants that could potentially affect ocular surface health, comfort and compliance.
We’ve all witnessed the effects of ocular surface disease in glaucoma and the consequences of long-term comorbidity. We can, and should, do everything in our power to address this growing problem. It’s up to us to introduce patients to the treatment possibilities that can address their glaucoma without compromising the ocular surface.2
Note: Dr. Karpecki consults for companies with products and services relevant to this topic.
| 1. Stewart WC, Stewart JA, Nelson LA. Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res. 2011;36:391-8. 2. Lee JH, Amoozgar B, Han Y. Minimally invasive modalities for treatment of glaucoma: an update. J Clin Exper Ophthalmol. 2017;8:4. 3. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. 4. Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON Study. Ophthalmology. 2019;126(1):29-37. 5. Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785-93. 6. Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. The Ocular Surface. 2017;15(3):511-38. 7. Liang H, Pauly A, Riancho L, et al. Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br J Ophthalmol. 2011;95(6):869-75. 8. Arici MK, Arici DS, Ozec AV, et al. Apoptotic effects of topical antiglaucoma medications on conjunctival epithelium in glaucoma patients. Eur J Ophthalmol. 2014;24(1):63-70. 9. Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol. 2007;18(2):134-39. 10. Agnifili L, Fasanella V, Costagliola C, et al. In-vivo confocal microscopy of meibomian glands in glaucoma. Br J Ophthalmol. 2013;97:343-49. 11. Arita R, Itoh K, Maeda S, et al. Effects of long-term topical anti-glaucoma medications on meibomian glands. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(8):1181-5. 12. Kim JH, Shin YU, Seong M, et al. Eyelid changes related to meibomian gland dysfunction in early middle-aged patients using topical glaucoma medications. Cornea. 2018;37(4):421-25. 13. Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, et al. The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J Glaucoma. 2016;25(9):770-4. 14. Arita R, Itoh K, Maeda S, et al. Effects of long-term topical anti- glaucoma medications on meibomian glands. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1181-85. 15. Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45(5):1360-8. 16. Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with sofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea. 2008;27(3):339-43. 17. Russ HH, Costa VP, Ferreira FM, et al. Conjunctival changes induced by prostaglandin analogues and timolol maleate: a histomorphometric study. Arq Bras Oftalmol. 2007;70(6):910-6. 18. Mastropasqua L, Agnifili L, Fasanella V, et al. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013;91(5):e397-405. 19. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618-21. 20. Lee S, Kim MK, Choi HJ, et al. Comparative cross-sectional analysis of the effects of topical antiglaucoma drugs on the ocular surface. Adv Ther. 2013;30:420-9. 21. Saade CE, Lari HB, Berezina TL, et al. Topical glaucoma therapy and ocular surface disease: a prospective, controlled cohort study. Can J Ophthalmol. 2015;50:132-6. 22. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556-63. 23. Kocabeyoglu S, Mocan MC, Bozkurt B, Irkec M. Effect of artificial tears on automated visual field testing in patients with glaucoma and dry eye. Can J Ophthalmol. 2013;48(2):110-4. 24. Kaštelan S, Tomic M, Metež Soldo K, Salopek-Rabatic J. How ocular surface disease impacts the glaucoma treatment outcome. BioMed Research International. 2013;2013:696328. 25. Rees G, Chong XL, Cheung CY. Beliefs and adherence to glaucoma treatment: a comparison of patients from diverse cultures. J Glaucoma. 2014;23(5):293-8. 26. Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015 May;9:833-41. 27. Sedlak L, Zych M, Wojnar W, Wygl dowska-Promie ska D. Effect of topical prostaglandin F2 analogs on selected oxidative stress parameters in the tear film. Medicina (Kaunas). 2019 Jul;55(7). 28. Holló G, Katsanos A, Boboridis KG, et al. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78(1):39-64. 29. Pfennigsdorf S, Eschstruth P. Preservative-free glaucoma treatment : Selection of the correct treatment in 1 min. Ophthalmologe. 2016;113(5):409-15. |

