 |
Peripheral Retinal Imaging and Disease Assessment
Using the right tools can help differentiate lesions and degenerations in this region.
By Julie Rodman, OD, MSc, Veronica Acuña, OD, and Pooja Alloju, OD
Release Date: February 15, 2022
Expiration Date: February 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss peripheral retinal imaging and disease assessment.
- Use OCT imaging to differentiate peripheral retinal lesions and degenerations.
- Recognize what these conditions look like on OCT imaging and other observational techniques.
- Review what conditions can arise in the peripheral retina.
- Diagnose peripheral retinal lesions and initiate management.
Target Audience: This activity is intended for optometrists engaged in managing patients with peripheral retinal disease.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Julie Rodman, OD, MSc, Veronica Acuña, OD, and Pooja Alloju, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #123219 and course ID 76574-TD. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Authors: Dr. Rodman receives consulting fees from Optovue, Maculogix and iCare. Drs. Acuña and Alloju have no financial interests to disclose. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
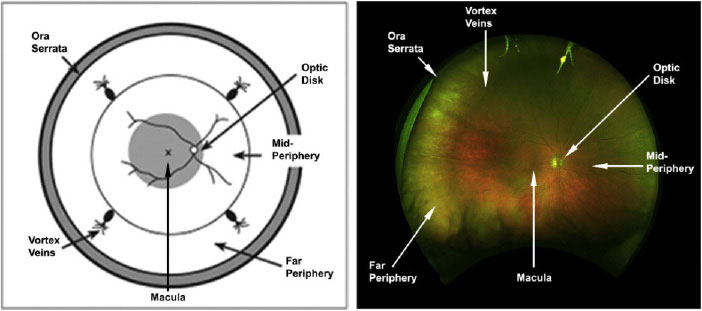 |
| Major landmarks in the retina.30,31 Click image to enlarge. |
Retinal imaging has dramatically evolved since the advent of the first ophthalmoscope in 1851.1 Advances in diagnostic technology have led to a broader understanding of retinal and choroidal disease and have provided new and innovative ways of evaluating the retina. Early imaging modalities helped capture the posterior pole; however, imaging of the retinal periphery was not viable. Diagnostic advances have since allowed for adequate visualization of the retinal periphery, which has become essential in the screening, diagnosis, monitoring and treatment of vision-altering diseases.
To establish consistent nomenclature regarding imaging of the retinal periphery, the International Widefield Imaging Study Group published guidelines on “widefield” and “ultra-widefield (UWF)” peripheral retinal imaging. Widefield is defined as a single-capture image centered on the fovea that includes the retina in all four quadrants posterior to and including the vortex vein ampullae.
UWF imaging is a single-capture view of the retina in the far periphery in all four quadrants.2 Standard imaging solely encompasses the posterior pole, retinal blood vessels, macula and optic nerve head. The introduction of UWF imaging has enabled image capture into the far periphery and has brought on a renewed interest in peripheral retinal abnormalities and their impact on various disease entities.
UWF imaging encompasses various modalities, including color fundus photography (using true color laser/confocal scanning laser ophthalmoscopy), UWF optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein angiography (FA) and indocyanine green (ICG) angiography. To maximize interpretation of these modalities, a basic understanding of retinal anatomy is necessary.
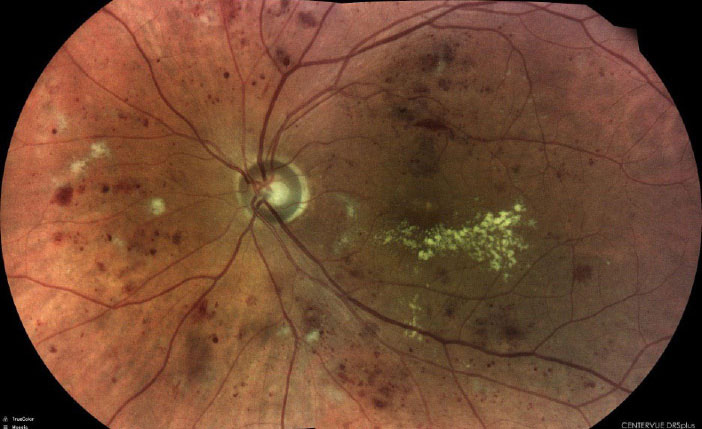 |
| Severe nonproliferative diabetic retinopathy captured with the DRSplus (iCare). Click image to enlarge. |
Retinal Anatomy Refresher
As we know, the retina is divided into various regions, including the posterior pole, equator and periphery. The posterior pole is made up of the optic nerve head, retinal vascular arcades and macula including the foveola, fovea, parafovea and perifovea.3 These designations are essential for retinal image analysis.
The foveola, the innermost region of the macula, is located at the center of the fovea where cone photoreceptors are located. It has a diameter of 0.35mm and contains the highest density (50 cones per every 100µm), allowing for a greater degree of resolution of visual acuity.4 The parafovea surrounds the fovea and the perifovea surrounds the parafovea.
The peripheral retina is divided into four distinct zones: the near periphery, middle periphery, far periphery and extreme periphery or ora serrata. The near periphery is defined as a 1.5mm ring adjacent to the 6mm diameter macula, the middle periphery is the next 1.5mm ring, the far periphery measures the next 9mm to 10mm temporally and 16mm nasally and the ora serrata measures the last 2.1mm temporally and 0.7mm nasally.5 The vitreous base is a 3mm to 6mm diameter area that borders the ora serrata and is the site where the collagen fibrils of the vitreous firmly attach to the internal limiting membrane of the peripheral retina.6
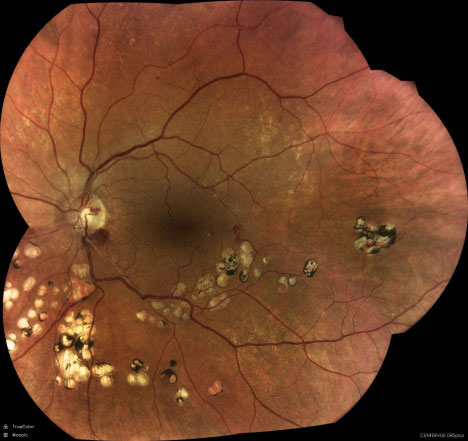 |
| Inferior retinal vein occlusion seen with the DRSplus (iCare). Click image to enlarge. |
History of Peripheral Retina Evaluation
Dilated fundus examination with use of both contact (i.e., three-mirror Goldmann lens) and non-contact fundus lenses of various powers along with binocular indirect ophthalmoscopy has historically been the standard for evaluation of the retinal periphery. Adjunct use of scleral depression is case-specific and recommended in certain scenarios such as those involving photopsia and floaters requiring a thorough exam of the vitreous base.
Scleral depression enables a greater field of view and provides a different retinal profile of suspicious areas that may reveal subclinical or subtle retinal breaks. Accurate descriptions of peripheral retinal findings were necessary, and detailed retinal drawings were used for documentation.
Imaging Modalities
A comprehensive understanding of when and how to use the different imaging modalities is key to ensure accurate diagnosis and effective disease management.
Fundus photography. Retinal photography is used as a screening device as well as a tool to aid in the detection, documentation and management of disease. Traditional fundus cameras allow for visualization of the posterior pole but provide limited views of the periphery. They have a 75° field of view with the use of eccentric fixation. Serial images can be obtained and montaged together to increase the field of view. However, montaging may result in distortion with magnification or minification of image detail.
In 2000, the Optomap (Optos) was introduced into the ophthalmic market. It is a non-contact camera that allows for a 200° field of view that covers 82.5% of the retinal surface. This technology uses a scanning laser technology with an ellipsoid mirror that allows for imaging of the retinal periphery. The use of red and green lasers provides a pseudocolor retinal image that is easily discernible from a traditional retinal camera, which uses a white light. Optomap units can be equipped with adjunct imaging modalities such as FAF, UWF OCT, ICG angiography and FA, which all enhance the imaging capability of peripheral retinal anomalies.
Other widefield imaging units include the Clarus 500 (Zeiss), the Eidon (iCare) and the Spectralis (Heidelberg). The Clarus 500 is an UWF imaging instrument that uses three wide-spectrum LEDs to enable image capture in true color. It captures 133° with one capture or 200° with the auto-montage feature. The Eidon is a non-mydriatic UWF confocal fundus imaging system that allows for multiple imaging modalities including TrueColor, blue, red, red-free and infrared. It captures 120° on a single shot and up to 200° using the mosaic feature.
The Spectralis delivers 55° confocal scanning laser imaging that includes infrared reflectance, FAF and multicolor imaging, acquired by obtaining infrared, green and blue reflectance images and applying a false color scheme. Scanning laser imaging provides increased image resolution over white light photography. It allows for visualization at different layers of the retina with different filters; thus, to image choroidal pathology or superficial retinal lesions, the red/green channel can be used. In this way, these fundus photography systems have become even more diagnostic in nature than ever before.
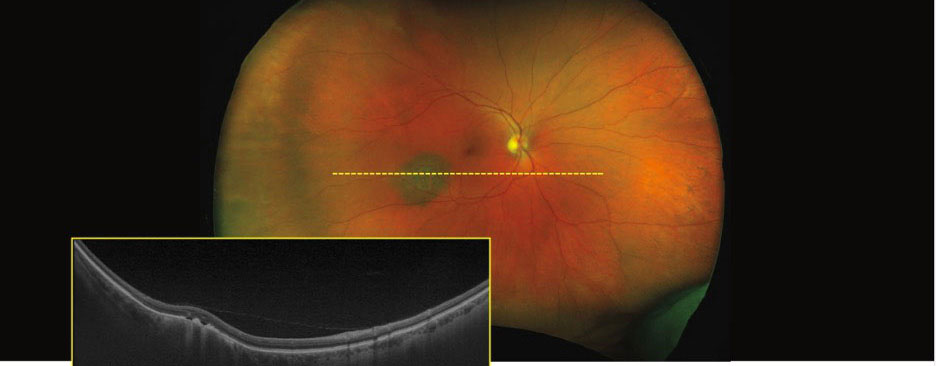 |
| UWF pseudocolor Optos image with corresponding OCT scan showing a slightly elevated choroidal nevus with overlying drusen within the inferior temporal arcades of the right eye. Image: Optos. Click image to enlarge. |
OCT. Spectral-domain OCT (SD-OCT) is a diagnostic modality that provides noninvasive, cross-sectional views of the retina, choroid, optic nerve and surrounding structures. SD-OCT includes a wide range of scan protocols that can maximally capture any area of the posterior pole including the vitreoretinal interface. It has been instrumental in advancing and enhancing our ability to diagnose a variety of conditions and execute appropriate management decisions based on the diagnosis. In fact, this technology has become a pillar of posterior segment evaluation along with dilated fundus ophthalmoscopy. However, its application and use in peripheral retinal imaging has been limited, bypassing the site of many vision-threatening pathologies.
The introduction of UWF SD-OCT has provided a new, innovative way of viewing the peripheral retina. UWF SD-OCT allows for precise visualization of peripheral anatomical features and can be extremely impactful in differentiating between various lesions, which in turn helps to guide subsequent management decisions. Entities such as retinal tears, holes with or without lattice degeneration and retinal detachments (RDs) are just a few examples of peripheral retinal entities that can be captured using UWF SD-OCT. Identifying subtle features and visualizing the full extent of these conditions is beneficial in determining which patients need to be referred and which can be monitored. Although not a replacement for clinical or funduscopic observation, it can serve as an excellent adjunct to examination.
UWF SD-OCT can be further used in evaluating peripheral choroidal and retinal pigment epithelium (RPE) lesions, such as congenital hypertrophy of the RPE, and determining malignant potential. When differentiating between choroidal nevi and melanomas, features suggestive of malignancy including anterior-posterior thickness >2mm with ultrasound, subretinal fluid and overlying retinal changes, such as to the photoreceptors or lipofuscin deposition, can be identified on simultaneous UWF SD-OCT capture and fundus imaging.7,8
Disruption to the overlying photoreceptors in the form of “shagginess” or irregularity may be an indication that the choroidal lesion has infiltrated the subretinal space. Similarly, lipofuscin—a hyperreflective substance anterior to the RPE on OCT—is a harbinger of malignancy. In contrast, the presence of drusen overlying a suspicious lesion is a sign more consistent with benign nevi and is an indication of chronicity. UWF SD-OCT is not only helpful in diagnosing these lesions but also in monitoring for progression.
 |
| UWF pseudocolor Optos image with corresponding OCT scan showing a far peripheral tear temporally. There is a visible break in the neurosensory retina with subretinal fluid within the lesion. Image: Optos. Click image to enlarge. |
FAF. This is another modality used to evaluate retinal peripheral abnormalities. It is a noninvasive imaging technology that evaluates the metabolic activity of the photoreceptor cells and the RPE. FAF employs a light source of a specific wavelength to excite intrinsically fluorescent lipofuscin.9 The intensity of autofluorescence depends on the amount of lipofuscin present. Eyes without pathology naturally emit a diffuse background autofluorescence except at the fovea, optic nerve head and retinal blood vessels. Increased autofluorescence is the result of excessive build-up of lipofuscin and is seen in diseases such as Stargardt’s disease, pattern dystrophy, Best’s disease and adult-onset vitelliform dystrophy.
FAF is extremely useful in evaluating various conditions affecting the posterior pole, including optic disc drusen, age-related macular degeneration (AMD), Stargardt’s and choroidal tumors, as well as in peripheral retinal diseases such as retinitis pigmentosa, choroideremia and cone/cone-rod dystrophy.9
Retinal angiography. Other invasive imaging platforms, such as fundus FA and ICG angiography, are remarkable technologies that remain at the forefront of retinal diagnostic imaging. These employ a water-soluble dye to visualize retinal and choroidal vasculature. ICG angiography uses a longer wavelength dye for better ocular penetration, making it the best modality to image the choroidal vasculature.10 The introduction of UWF FA and UWF ICG angiography has allowed for the same level of visualization of the retinal periphery that we have grown accustomed to seeing in the posterior pole and equatorial regions.
Evaluation of widefield angiography in various diseases has proven beneficial, as patients without clinically visible peripheral retinal disease may manifest peripheral vascular variations that may alter the course of the disease.11 When comparing highly myopic eyes with emmetropic eyes, the former have large areas of capillary nonperfusion, telangiectasia and microaneurysms on widefield FA.12
The utility of widefield FA has also been studied in AMD. The presence of peripheral leakage on angiography has been positively correlated with neovascular AMD development.13 AMD is thus a panretinal disease as it manifests in the posterior pole but also affects the peripheral retina.
Widefield angiography has also become extremely beneficial in managing diabetic patients. In diabetes, widefield FA images capture 3.9x more areas of retinal nonperfusion, 1.9x more retinal neovascularization and 3.2x more retinal surface area than the standard ETDRS map.14 In addition, late leakage of peripheral retinal vessels in poorly perfused areas is correlated with the development of macular edema and neovascularization.
The presence of predominantly peripheral lesions in diabetic eyes is predictive of a threefold increased risk of diabetic retinopathy progression and a nearly fivefold increased risk of developing proliferative retinopathy.15 These lesions correlate with areas of peripheral nonperfusion on widefield angiography and are thus linked to an increased risk of retinopathy progression.16 Since these lesions are not readily visible in the posterior pole, it is essential to perform a peripheral retinal exam to gain insight into the disease course. Patients with greater areas of retinal nonperfusion had better outcomes from anti-VEGF therapy, including improvements in macular thickness and visual acuity.17 UWF imaging with FA also highlights abnormalities seen in noninfectious uveitis or vasculitis that may have been missed on standard fundus exam.18
 |
| Inferotemporally located retinoschisis with corresponding OCT scan in the left eye. OCT displays the schitic cavity separating the inner retinal layer from the outer retinal layers. Image: Optos. Click image to enlarge. |
Conditions to Look Out For
Lattice degeneration is a common peripheral retinal degeneration, with a prevalence of 7% to 10% in the general population.19 It is characterized by localized retinal thinning, vitreoretinal adhesion at the borders and overlying vitreous liquefaction. Lattice degeneration can vary widely in appearance; however, it is usually round, oval or elongated, with the long axis parallel to the ora serrata. It can also appear spindle-shaped and have associated sclerosed vessels. It is commonly seen bilaterally, more so in the superior and inferior quadrants.
In most cases, lattice can be adequately visualized using binocular indirect ophthalmoscopy. Often, the vitreoretinal traction is difficult to appreciate on peripheral retinal ophthalmoscopic examination and is more readily visible using UWF SD-OCT.
Characteristics of peripheral lattice degeneration on UWF SD-OCT include retinal thinning, retinoschisis, vitreous liquefaction anterior to the thinned retina and retinal breaks or atrophic holes with or without subretinal fluid.20 Retinal holes within the lattice will manifest a full-thickness loss of retinal tissue with or without subretinal fluid adjacent to the hole. Peripheral atrophic holes not associated with lattice may also present with overlying operculum either attached, partially attached or completely detached. In instances of a partially attached operculum, hyper-reflective vitreous fragments may be visible surrounding the operculum.21
Senile (degenerative or acquired) retinoschisis is a commonly encountered peripheral retinal pathology with a prevalence of approximately 3.8%.22 It presents clinically with microcystoid degeneration and/or separation of the neurosensory retina, resulting in an immobile, transparent bullous or smooth inner retinal elevation.23
RD is a clinically similar entity to retinoschisis. While sight-threatening, RD has an incidence of approximately one in 10,000.24 Normally, the RPE maintains a tight junction with the overlying neurosensory retina; however, when exposed to stressors, this tight barrier deteriorates, resulting in an accumulation of subretinal fluid between the neurosensory retina and the RPE and causing an RD.
 |
| UWF pseudocolor Optos photography with corresponding OCT scan showing a chronic peripheral RD. OCT illustrates the separation between the neurosensory retina and underlying RPE. Serial OCT scans allow for full visualization of the lesion. Image: Optos. Click image to enlarge. |
RDs can be characterized as one of four types: rhegmatogenous, tractional, exudative or combined tractional-rhegmatogenous. Rhegmatogenous RDs usually occur due to a retinal tear brought on by a preceding event, i.e., a posterior vitreous detachment or trauma. Tractional RDs occur because of proliferation at the vitreoretinal interface creating tractional points that can pull on the neurosensory retina creating a separation. Tractional detachments occur in cases of proliferative diabetic retinopathy or proliferative sickle cell retinopathy, among others. Exudative RDs are seen because of subretinal fluid accumulation secondary to localized inflammation or exudative extravasation from a lesion.
It is often challenging to differentiate a retinal detachment from senile retinoschisis. RDs can vary in appearance due to their wide array of etiologies, but rhegmatogenous RDs often present similarly to retinoschisis as smooth or bullous elevations. However, RDs are associated with a visible retinal break. Pigment demarcation lines may form at the edge of the detached/attached retina in chronic detachments, but they do not form at the edge of a retinoschisis.25
Scleral indentation and biomicroscopic evaluation are useful but self-limiting and not always sufficient. Scleral indentation will flatten an RD as the subretinal fluid will be displaced through the retinal break into the overlying vitreous.26 Due to absence of a break with retinoschisis, this presentation will not flatten on scleral depression. It is often difficult to obtain an adequate view of the lesion with scleral depression, resulting in a clinical conundrum. Adjunct use of UWF SD-OCT in these scenarios may prove to be extremely beneficial.
UWF SD-OCT renders a cross-sectional image of these lesions, providing a view of the retinal anatomy that aids in the diagnosis and differentiation of the lesions. On UWF SD-OCT, senile retinoschisis will present with hyporeflective cystoid cavities and columns, creating a sawtooth-like schisis or splitting of the inner nuclear and outer plexiform layers of the retina.27 OCT of an RD will display a full-thickness, fully detached neurosensory retina from the underlying RPE. A senile retinoschisis will exhibit attenuation of the inner retina and a splitting of the retinal layers at the inner nuclear layer-outer plexiform layer junction. At the edge of the lesion, fibers may extend across the schisis cavity connecting the inner and outer neurosensory retina. Often, the lesion quickly becomes highly elevated and bullous as you move into the lesion with a total separation of the outer from the inner retina. The outer retina remains in opposition to the RPE unlike in an RD.
UWF SD-OCT can help reduce the referral rates for common clinical findings such as white without pressure (WWOP) and dark without pressure (DWOP). WWOP is a commonly encountered peripheral retinal finding often found in young, myopic patients.28 It is characterized by an area of retinal whitening found in the far periphery that blocks the view of the underlying choroid. Exact etiology is unknown; however, it is thought to be due to an anomalous vitreoretinal relationship. While these are relatively benign conditions, they are frequently misdiagnosed for the more pathologic RD or retinoschisis. Due to the similar appearance of these entities, scleral depression alongside UWF SD-OCT can be performed to determine if the retina is intact.
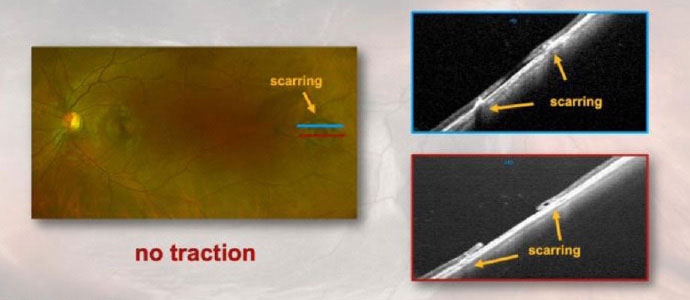 |
| Optos photography and corresponding OCT showing a temporal retinal hole with scarring but without surrounding subretinal fluid cuff in the left eye. Scarring from previous laser is seen as distinct areas of hyperreflectivity adjacent to the retinal hole (absence of neurosensory retina). Image: Optos. Click image to enlarge. |
Unlike RDs or retinoschisis, OCT of WWOP and DWOP will reveal a flat lesion without lifting of the neurosensory retina from the RPE, as in an RD, or splitting of the retinal layers, as in a retinoschisis. On OCT, these lesions are correlated with an abrupt change of the photoreceptor reflectivity, with relative hyporeflectivity of photoreceptor zones (ellipsoid and interdigitation zones, as well as outer segments) within the dark lesions and relative hyperreflectivity within the white lesions.29
Peripheral cystoid degeneration is another entity that is well captured with peripheral retinal imaging and UWF SD-OCT. Peripheral cystoid degeneration consists of microcystic changes that are present in adult eyes. They are found in the ora serrata and expand posteriorly and circumferentially, increasing as a person ages. More specifically, they are closely packed cystic areas in the inner nuclear and outer plexiform layers of the retina with indistinct boundaries. They are typically benign, hazy-gray lesions with depressions or cysts but can also lead to a degenerative type of retinoschisis. On SD-OCT, peripheral cystoid degeneration will present with hyporeflective cystoid cavities and columns creating a sawtooth pattern. Many of these cavities and columns span the entire thickness of the neural retina.21
Takeaways
Optometrists play a pivotal role in eye care and can alter the course of myriad sight-threatening diseases our patients suffer from. Visualizing the retinal periphery through the integration of widefield imaging modalities allows for early detection and optimizes the overall prognosis in a variety of conditions. Integrating these technologies into our everyday practice elevates the level of care that we can provide and puts us at the forefront of our profession.
Dr. Rodman is chief of the Broward Eye Care Institute in Fort Lauderdale, FL. Her research interests include OCT/OCT-A and vitreoretinal disease. She receives consulting fees from Optovue, Maculogix and iCare. Dr. Acuña graduated from Nova Southeastern University’s (NSU) College of Optometry in 2021, at which point she started her residency at NSU in primary care with an emphasis on ocular disease. Dr. Alloju graduated from Southern College of Optometry in 2021 and is currently pursuing her dual primary care and cornea and contact lens residency at NSU. Drs. Acuña and Alloju have no financial interests to disclose.
1. Keeler CR. The ophthalmoscope in the lifetime of Hermann von Helmholtz. Arch Ophthalmol. 2002;120:194-201. 2. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the international widefield imaging study group. Ophthalmol Retina. 2019;3:843-9. 3. Bringmann AS, Syrbe K, Gorner J, et al. The primate fovea: structure, function, and development. Prog Retin Eye Res. 2018;66:49-84. 4. Rehman I, Mahabadi N, Motlagh M, et al. Anatomy, Head and Neck, Eye Fovea. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. 5. Duke-Elder SW. System of Ophthalmology, Vol. 11. The Anatomy of the Visual System. St. Louis: Kimpton Publishers; 1976. 6. Hogan MJ. The vitreous, its structure, and relation to the ciliary body and retina. Proctor award lecture. Invest Ophthalmol. 1963;2:418-45. 7. Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol. 2002;13:135-41. 8. Muscat S, Parks S, Kemp E, et al. Secondary retinal changes associated with choroidal nevi and melanomas documented by optical coherence tomography. Br J Ophthalmol. 2004;88:120-4. 9. Yung M, Klufas MA, Sarraf D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retin Vitr. 2016;2:12. 10. Staurenghi G, Bottoni F, Giani A. Retina (Fifth Edition). Clinical Applications of Diagnostic Indocyanine Green Angiography. WB Saunders; 2013. 11. Shah AR, Abbey AM, Yonekawa Y, et al. Widefield fluorescein angiography in patients without peripheral disease: a study of normal peripheral findings. Retina. 2016;36(6):1087-92. 12. Kaneko Y, Moriyama M, Hirahara S, et al. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55(3):1432-9. 13. Madhusudhan S, Beare N. Wide-field fluorescein angiography in wet age-related macular degeneration. Scientific World J. 2014:536161. 14. Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-8. 15. Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949-56. 16. Kimble JA, Brandt BM, McGwin G, Jr. Clinical examination accurately locates capillary nonperfusion in diabetic retinopathy. Am J Ophthalmol. 2005;139(3):555-7. 17. Singer M, Tan CS, Bell D, et al. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34(9):1736-42. 18. Hong BK, Khanamiri HN, Rao NA. Role of ultra-widefield fluorescein angiography in the management of uveitis. Can J Ophthalmol. 2013;48:489-93. 19. Flaxel CJ, Aldmen RA, Lim JI, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration PPP 2019. American Academy of Ophthalmology. 20. Tsai CY, Hung KC, Wang SW, et al. Spectral-domain optical coherence tomography of peripheral lattice degeneration of myopic eyes before and after laser photocoagulation. J Formos Med Assoc. 2019;118:679-85. 21. Choudry N, Golding J, Manry MW, et al. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123:1368-74. 22. Buch H, Vinding T, Nielsen NV. Prevalence and long-term natural course of retinoschisis among elderly individuals: the Copenhagen City Eye Study. Ophthalmology. 2007;114:751-5. 23. Madjarov B, Hilton GF, Brinton DA, et al. A new classification of the retinoschisis. Retina. 1995;15:282-5. 24. Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100(2):289-92. 25. DiSclafani M, Wagner A, Humphrey W, et al. Pigmentary changes in acquired retinoschisis. Am J Ophthalmol. 1988;105:291-3. 26. Shukla SY, Batra NN, Ittiara ST, et al. Reassessment of scleral depression in the clinical setting. Ophthalmology. 2015;122(11):2360-1. 27. Stehouwer M, Tan SH, van Leeuwen TG, et al. Senile retinoschisis versus retinal detachment, the additional value of peripheral retinal OCT scans (SL SCAN-1, Topcon). Acta Ophthalmol. 2014;92(3):221-7. 28. Zhang T, Wei YT, Huang WB, et al. Prevalence and characteristics of peripheral myopic retinopathy in Guangzhou office workers. Int J Ophthalmol. 2018;11(8):1390-5. 29. Fawzi AA, Nielsen JS, Mateo-Montoya A, et al. Multimodal imaging of white and dark without pressure fundus lesions. Retina. 2014;34(12):2376-87. 30. Santini B. Controlling Myopia in Children. Review of Myopia Management. 2019. 31. Sasaki H, Jonasson F, Kojima M, et al. The Reykjavik Eye Study—prevalence of lens opacification with reference to identical Japanese studies. Ophthalmologica. 2000;214(6):412-20. |
