Neuro-Ophthalmic Disease Basics: Evaluating the Efferent Visual System
Accurately diagnosing neuro-ophthalmic disease can be difficult and intimidating. But a stepwise approach can make the task less daunting and keep you on track.
Goal Statement:
Many optometrists are uncomfortable with cases of neuro-ophthalmic disease. This article will help you not only become more confident at diagnosing and monitoring neuro-ophthalmic disease of the efferent visual system, but also feel more confident in referring and comanaging a patient with a specialist.
Faculty/Editorial Board:
Dr. Malloy is the director of the Neuro-Ophthalmic Disease Service at Pennsylvania College of Optometry, Salus University, and clinical assistant professor of neurology at Hahnemann/Drexel College of Medicine in Philadelphia.
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID is 44084-NO. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Dr. Malloy has no relationships to disclose.
Many optometrists feel uncomfortable with cases related to neuro-ophthalmic disease. Either you fear that you'll overlook an important finding and not refer a patient who truly does have neuro-ophthalmic disease, or you're concerned that you'll over-analyze a certain symptom and refer a patient for unnecessary consultation or testing.
What you should realize is that you already have all of the required tools to accurately examine and assess a patient who has a neuro-ophthalmic disease process. All you need now is to spend some time sharpening those tools.
Previous articles have reviewed the afferent visual system. Here's the counterpart approach for assessing the efferent visual system.
It's impossible to include all aspects of the efferent visual system evaluation in one article. However, this article covers the important basics that will help you not only become more confident at diagnosing and monitoring neuro-ophthalmic disease of the efferent visual system, but also feel more confident in referring and comanaging a patient with an appropriate specialist.
Afferent vs. Efferent
As referenced in the June 2008 article regarding the afferent visual system, separating your examination into tests that assess the afferent versus efferent visual system can make neuro-ophthalmic disease less overwhelming.1 It is important to make this distinction so that you know which tests to do when checking for a certain disease process. Granted, a disease process may affect both systems. However, if you think of the afferent and efferent systems separately, it will be easier to determine which tools and tests to employ to make a proper diagnosis.
The afferent system carries impulses from the eye to the central nervous system, whereas the efferent system carries impulses from the central nervous system to the eye. Therefore, the afferent system deals with optic nerve function. The efferent system is associated with ocular motility issues, ptosis or eyelid retraction, anisocoria and nystagmus.
When dealing with any issue involving pupil sizes, eyelids or eye movements, you must know how to test all measures of the efferent visual system. Many times, a disease process that affects the efferent visual system can manifest through a combination of findings related to eyelid position, pupil size and ocular motility. This could, therefore, indicate possible involvement of a combination of muscles, the neuro-muscular junction, various nerves (cranial nerves or autonomic nervous system) and/or the brain/brainstem/ cerebellum. A good understanding of neuro-anatomy is critical in localizing a disease process. In addition, a cursory neurologic examination assessing all cranial nerves, motor function, sensory function and coordination is also helpful in localizing a lesion.
Patient History
Just as patient history is a key factor in assessing the afferent visual system, it's also essential in assessing the efferent visual system. You must consider the patient's systemic and ocular health, as well as lifestyle habits.
How to Rule Out Horner Syndrome
An in-office diagnostic test for Horner syndrome is the use of readily available apraclonidine ophthalmic solution. Instill either 0.5% or 1% apraclonidine into both eyes and assess the pupil sizes for change. A positive result is a dilation of the smaller pupil, and therefore a reversal of anisocoria. The eye with the Horner syndrome dilates due to upregulation of the alpha-1 receptors. If the test remains negative at 30 minutes, you need to repeat the measurements at one hour. Note that a false negative result may occur with acute Horner syndrome, such as in acute internal carotid artery dissecton.6-8 In determining the urgency of a workup, an important reminder is that Horner syndrome with any pain in the neck or head region needs to be considered a carotid dissection until that can be ruled out. This is a medical emergency because of its risk of impending stroke; send the patient to the emergency room for urgent evaluation and treatment. 9 |
• Systemic history. Important aspects of the systemic history include any condition that can contribute to an ischemic, inflammatory or infectious process. Be sure to ask about specific disease states such as multiple sclerosis, myasthenia gravis, thyroid disease, stroke, systemic infections or inflammations, or any other known health conditions. Also, any history of cancer is important.
If there is a history of cancer, always consider that recurrence or a metastatic lesion could be the cause of the clinical presentation.
Additionally, if the patient has had a stroke or other intracranial pathology in the past, you must obtain a copy of the imaging report to determine the location of the abnormality. If it's not in an anatomic region that would explain the efferent visual system findings, additional work-up is needed to find the cause.
• Ocular history. Clearly, ocular history is important in determining the differential diagnoses for an efferent visual system issue. Find out if the patient has a history of trauma, which could contribute to changes in pupil size and shape, eyelid positioning, ocular alignment and motility. Often, patients may either forget about or deny a history of trauma to their eyes or head, but then on external evaluation you'll note scars or other telltale signs of past trauma or surgery. However, don't assume that an efferent visual system issue is a result of past trauma unless you have substantive proof from previous records or examinations, or until other etiologies have been ruled out.
• Medications. Know all medications and supplements your patient is using. Some medications can contribute to muscle weakness and therefore contribute to ptosis or ocular motility problems. For example, statins have been proven to cause diplopia.2 Anti-seizure medications such as phenytoin can cause nystagmus.3
• Social history. Because substance abuse could be related to the patient's condition, ask about the patient's history of tobacco, alcohol and drug use. All of these can be risk factors for stroke. In addition, tobacco use increases the likelihood of ocular involvement from thyroid disease, and alcohol use can cause nystagmus.4,5
Assessment Tools
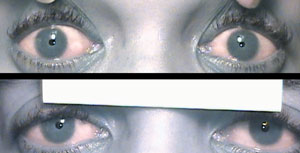
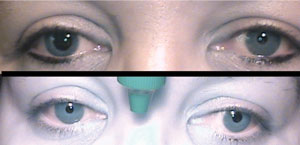 Diagnostic drops for pupil testing: pre-drop presentation (top) is anisocoria OD>OS, which was greatest in bright illumination, signifying a parasympathetic issue. Differential includes CN III, pharmacologic dilation, tonic pupil. After instillation of 0.12% pilocarpine (bottom), constriction OD confirms a tonic pupil. |
The main tools needed to evaluate efferent visual function are pupil measurements, eyelid measurements, exophthalmometry, ductions and versions, as well as cover testing. You're already familiar with these tools, and likely use them regularly. However, you need to be sure you're using them to their maximum capacity. Often, efferent visual system problems can affect multiple components of the efferent visual system. Therefore, no value or measurement should be assessed in isolation. For example, whenever you note a difference in pupil sizes, also keep in mind the results of eyelid measurements and ocular motility findings. Such is the case when considering a Horner syndrome or cranial nerve (CN) III palsy.
• Measuring pupil sizes. Too often, doctors simply estimate or “eyeball” pupil sizes; we need to be sure to measure them as accurately as possible. Sometimes critical—even life-threatening—diagnoses are made because the difference in pupil sizes is 0.5mm or less.
For all pupil measurements, be sure the patient is looking at a distant target. If the patient looks at a near target, you'll confuse the reaction to light with the accommodative response.
If the pupils are the same size, or isocoric, there is no efferent pupil problem. If the pupils are anisocoric, the cause could be either physiologic or pathologic, and careful pupil measurements can help make that distinction. The best way to measure pupil size is with the half circle indicators. These half circles are present in 1mm increments. Of course, pupil sizes do not just come in 1mm steps.
Therefore, you must interpolate the pupil size to at least the nearest 0.5mm. This will be much harder to do using a millimeter rule.
• Pupil size in bright vs. dim illumination. Pupil sizes need to be measured in contrasting illuminations. They should be measured with the illumination as high as possible, and then again with the illumination as low as possible. To measure pupils in bright illumination, have the room and overhead lights on, with the light equally illuminating both eyes. When measuring pupils in dim illumination, all lights should be off. Use a transilluminator directed from below, with just enough illumination that you can see and measure the pupils. Be careful not to place more illumination on one eye than the other, or measure the pupils at two different times with unequal amounts of illumination. Try to hold the light at a comparable distance and angle from each eye. This will help to ensure consistency in measurements.
Take note of the difference in pupil sizes in bright illumination. Compare this to the difference in pupil sizes in dim illumination. If the difference in pupil sizes in each illumination is the same, this is consistent with physiologic anisocoria, and no additional work-up is needed.
One exception is if there is a ptosis on the same side as the smaller pupil, in which case you should still rule out Horner syndrome.
If the anisocoria is greater in either bright or dim illumination, this signifies a pathologic process of the autonomic nervous system. Because the sympathetic system acts to dilate the pupil, damage to the sympathetic pathway results in a smaller pupil and anisocoria that is greater in dim illumination.
This, especially in the setting of a smaller palpebral aperture on the same side, is indicative of Horner syndrome. Because there is a characteristic dilation lag in Horner syndrome, you'll note the anisocoria in dim illumination is greatest as soon as the lights are turned off, and somewhat less several seconds later. (See “How to Rule Out Horner Syndrome.”)
Conversely, because the parasympathetic system acts to constrict the pupil, damage to the iris sphincter leads to anisocoria, which is greater in bright illumination. When this is present, we must consider differentials, including a CN III palsy, pharmacologic dilation and tonic pupil. Of course, other tests of efferent visual function, such as eyelid measurements and ocular motilities, help in this distinction. Keep in mind that the pupil does not have to be fixed and dilated in a pupil-involved CN III palsy. It is possible to have a partial pupil-involved CN III palsy, which could manifest as anisocoria greater in bright illumination.
Differential Diagnosis for Pupillary Light-Near Dissociation
|
If you think the patient may have a CN III palsy, do not put any drops in the eye so that when the care is transferred to the emergency room, an accurate pupil assessment can be made. On the other hand, if you think the patient likely does have pharmacologic dilation of the pupil, diagnostic testing is helpful to prevent unnecessary work-up. If the patient's pupils are pharmaco-logically dilated, the receptors are already bound by the substance that is dilating the pupils. For this reason, if any concentration of pilocarpine is instilled, it will not constrict the pupil. If it really is a CN III palsy, the receptors are not occupied, and 1% pilocarpine would constrict the pupil.
In terms of using diagnostic drops to assess for a tonic pupil, use a weak concentration—0.125% pilo-carpine. To make it in the office, dilute the pilocarpine with the appropriate amount of saline. For example, you can place a drop of 1% pilocarpine into a contact lens case and then dilute it with eight drops from a single-use vial of non-preserved artificial tears and mix. Discard the remaining tears from the vial, which can now be easily used to suck back up the mixture and instill a single drop into the eye. Wait 30 minutes or up to one hour to check for a result. In this case, a positive result is constriction with this weak pilocarpine dosage, which would not constrict a normal eye.10 Be sure to assess the need to use diagnostic drops before doing tonometry because you should not touch the cornea prior to using these drops.
• Comparison of pupil reaction to light vs. pupil reaction to near. Assess pupillary reaction to light in each eye. If it is absent, or less than expected, be sure to check the pupil reaction to a near stimulus. This is a step that is often overlooked, and it can have great diagnostic potential. When testing for the accommodative response, have the patient hold up a finger in front of their nose and tap the finger to trigger the proprioceptive cues. Have them alternate from fixating on their finger to a distant target. Watch for the change in pupil size either from distance to near, or near to distance.
Compare this response with that to a light stimulus. Whenever the reaction to accommodation is greater than the reaction to light, there is presence of light-near dissociation. There are five main causes (see “Differential Diagnosis for Pupillary Light-Near Dissociation,” above), and careful testing of other aspects of the efferent visual system can help differentiate among these.11-16
• Eyelid measurements.
Main Causes of Ptosis
|
1. Palpebral apertures. Palpebral apertures need to be measured any time there is anisocoria and any time there is ocular misalignment. Assess palpebral apertures by measuring the distance between the lower lash line and the upper lash line while the patient is fixating on a distant target. It is essential that the frontalis muscle is held and immobilized during these measurements, so that the patient cannot raise their eyelids and possibly increase their palpebral aperture. Take note of any asymmetry—if asymmetry is noted, determine which eyelid is abnormal. Differentiate between ptosis in one eye vs. eyelid retraction in the fellow eye.
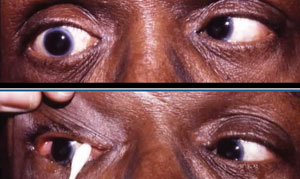
If you suspect myasthenia gravis, measure the palpebral aperture with fatigue and icepack testing. When assessing for fatigue, first measure pre-fatigue palpebral apertures. Then have the patient sustain upgaze for two minutes, being sure the patient is looking up and not moving their chin up. Watch for any lid droop during the two minutes. Even if no lid droop is noted, measure palpebral apertures again immediately after the two minutes of upgaze. Remember, be sure to hold the frontalis muscle; the patient should look at the same distance target used for the pre-fatigue measurements. Any decrease in palpebral aperture after the two minutes of sustained upgaze indicates fatigue, and could be consistent with myasthenia gravis.17
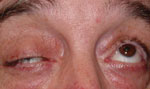 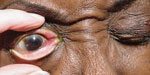 To assess for myasthenia gravis, check the lid function. The top photo shows lid droop with sustained upgaze/fatigue. Bottom photo shows orbicularis oculi weakness when prying lids apart. |
To further assess for myasthenia gravis, look for an increase in palpebral apertures after holding an icepack on the eyelids for two minutes. But first, measure pre-icepack palpebral apertures to assess for interval change immediately following the two minutes of eyelid cooling. An increase in palpebral aperture after the application of ice suggests myasthenia gravis.18-19
Assessing the strength of the orbicularis oculi muscles also helps in assessing for myasthenia gravis. Have the patient forcefully close their eyes; you should not be able to pry the eyelids open. If you can, this suggests weakness that can be a feature of myasthenia gravis.
2. Lid crease. In order to measure the lid crease, have the patient look down, and measure the distance from the upper lash line to any eyelid creases. There may be one or several creases in each eye. The lid crease signifies the insertion of the levator muscle. Asymmetric lid creases may signify disinsertion of the levator, which could be a cause of ptosis.
3. Levator function. In order to measure levator function, be sure the patient's head remains still during the testing. First, have the patient look down, and put the zero of the ruler in a position consistent with the upper lash line. Do not move the ruler. Now, ask the patient to look up as high as possible, and note where the upper lash line now intersects the ruler.
Most common muscles involved in thyroid eye disease:
|
This maximum excursion of the upper lid from extreme downgaze to extreme upgaze is the measurement of levator function. Since CN III innervates the levator muscle, a CN III palsy is an important cause of reduced levator function.
• Exophthalmometry. Problems with the efferent visual system can localize to the orbit, so it's important to measure exophthalmometry. Hertel exophthalmometry is superior to Luedde exophthalmometry because of the ability to measure the base, which signifies the distance between the lateral orbital rim in both eyes. Be sure to use the same base on all follow-up measurements to accurately assess for interval change. If you don't have an exophthalmometer, you can assess for ease of retropulsion of the globe, as well as have the patient lean forward with their face parallel to the floor to see if an asymmetry becomes more apparent with the help of gravity, as seen with an orbital varix or mass.
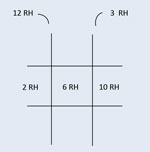
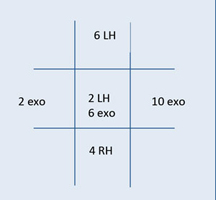
 This is an example of the documentation of a right abduction deficit, where the right eye moves out only 40% of normal capacity. The 100s represent 100% or full ductional capacity in all other positions of gaze. If the patient did not move in a particular direction from midline, a 0 is noted. 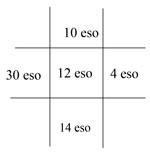 This is an example of the documentation of the cover test results on the same patient as above. There is an eso deviation, which increases in right gaze; this is consistent with a right abduction deficit. Note: If there were both a horizontal and vertical deviation, both would be noted in the same box. |
• Ductions and versions. Ductions and versions need to be performed, looking for even subtle limitations. Do versions first (eye movements with both eyes open). If versions are not 100% normal in each eye in every direction, then do ductions, assessing movements with each eye individually.
Comparing versions with ductions can help to differentiate a restrictive from a neurogenic cause of limited motility. In a restrictive process, such as thyroid eye disease or orbital mass, the degree of movement will be equal with ductions and versions. However, with a neurogenic etiology, such as a vasculopathic cause, the degree of movement will be better on ductions than on versions. Similarly, forced duction can also help in this regard. A restrictive process would demonstrate a positive forced duction test in which the eye cannot be moved into its full ductional range. A neurogenic process would demonstrate a negative forced duction test, in which the eye would be able to easily be moved into its full ductional range.20 To perform this test, anesthetize the eye, use a cotton swab placed on the limbus opposite the ductional limitation, and gently roll the eye in the direction of the ductional limitation. Avoid pushing the eye back in the socket, which can produce a false positive result.
The patient's full range of motion of each eye in all directions needs to be evaluated using a large circle so that every position of gaze is assessed—using an H pattern or a cross pattern can miss some positions of gaze. Make the circle large enough to assess full range of motion; with the arm completely outstretched and moved in a full circle.
Look for subtle limitations. In lateral gazes, that would be best detected by looking for complete burying of the sclera in abduction and adduction. Any subtle asymmetry can be important—there are times when a CN VI palsy presents with 90% or even 95% normal abduction. We need to compare the ductions with the cover test results and not rely on ocular motility assessment alone to diagnose a cranial nerve palsy. A patient who truly has a right abduction deficit will also have cover test results that get more eso on right gaze.
• Alternate cover test in nine positions of gaze. To do cover testing in different positions of gaze, have the patient remove his/her eyeglasses to avoid induced prismatic effect when the patient is looking in different positions of gaze. This means you may need to give them a larger target they can see without their glasses, if possible. If the patient cannot fixate a large target without their glasses, they may have to keep them on. Do cover testing in primary gaze. Then, turn the patient's head in different directions and have them continue to fixate on the distance target in the cardinal positions of gaze.
Neutralize the cover test using both horizontal and vertical prism bars as appropriate. Neutralize the largest component (horizontal or vertical) first, keep that prism bar in place, and then neutralize the remaining component with the prism bar over the other eye. Be sure to hold the prism bars in a position so that the patient is looking directly through them. This means that in different positons of gaze, the bars may not be sitting in the same plane as eyeglasses would sit.
 Forced duction testing moves the eye into full abduction; this is a negative test, indicating no restriction. This is not thyroid, but could be either myasthenia gravis, or a problem anywhere along the course of CN VI from pons to orbit. Forced duction testing moves the eye into full abduction; this is a negative test, indicating no restriction. This is not thyroid, but could be either myasthenia gravis, or a problem anywhere along the course of CN VI from pons to orbit. |
Remember, if there is a significant ductional limitation, you will need to put up the prism first in order to get the patient to find the target in the limited direction of gaze. Also, if there is a vertical misalignment, doing cover testing in both right and left head tilt helps to determine if the pattern is consistent with a CN IV palsy.
To differentiate between a CN IV palsy and a skew deviation, try assessing torsion with double Maddox rod testing. In a CN IV palsy, there may be mild excyclotorsion of the hyper eye, whereas in a skew deviation there may be incyclotorsion of the higher eye and excyclotorsion of the lower eye.21 Thyroid eye disease or myasthenia gravis can also mimic a CN IV palsy.22
 Pattern of a right CN IV palsy: Possible excyclotorsion <10 degrees if acute. No torsion likely if chronic. Torsion viewed by double Maddox rod testing or downward deviation of the macula. Hyperdeviation worse in contralateral gaze and ipsilateral head tilt. 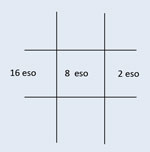 Pattern of a right CN VI palsy: Eso deviation greatest in ipsilateral gaze. (Pattern is the same for any abduction deficit, not just for CN VI palsy.) |
Record the cover test result on a grid so that you can assess the pattern for any potential CN palsy, or other recognizable diagnostic pattern.
• Assessing for nystagmus. Have the patient hold their fixation as steady as possible in each position of gaze. Note any rhythmic movements, which can be pendular or jerk. Jerk nystagmus is named for the fast phase. Nystagmus is otherwise classified based on the amplitude and frequency of its movements. Congenital nystagmus remains horizontal and symmetric. Any new, vertical or asymmetric nystagmus is concern ing for pathology. Neuroimaging is warranted in these cases. Nystagmus often localizes to the cerebellum, brainstem or vestibular system.
Further Assessing Efferent Findings
When you determine that a patient has an abnormality of the efferent visual system based on the above noted tools for measuring eyelids, pupils and ocular motilities, it is time to develop differential diagnoses.
• Work-up. When a problem is found with the efferent visual system, appropriate work-up and/or referral is warranted. Depending on your practice locale, the urgency of the condition and many other factors, you may opt to order the work-up yourself and/or refer to a neuro-ophthalmologist, neurologist or perhaps to the emergency room. The work-up typically includes neuroimaging and lab testing.
An MRI is not possible if the patient has a pacemaker or defibrillator, cochlear implants, a bullet or other metal in the body, or recent placement of stents. Other contraindications may include excessive weight (more than 350lbs.) and claustrophobia, which may preclude someone from having an MRI in a closed gantry. The contrast used in MRI is gadolinium, which causes relatively few adverse effects. However, facilities request a blood urea nitrogen (BUN) and creatinine level in patients over age 50 or with diabetes and hypertension to check for kidney problems. If the BUN or creatinine is elevated, use of gadolinium may be contraindicated because of the possibility of nephrogenic systemic fibrosis.23
 Pattern of a right CN III palsy: Possible right ptosis. Possible anisocoria greater in bright illumination. |
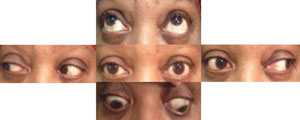 Partial, pupil-involved right CN III palsy. Note that the eye is not down and out. Instead, note the reversing hyperdeviation and greater exo across from the vertically limited eye, along with the mild right ptosis. This is a medical emergency—work-up is needed to rule out an aneurysm. |
If an MRI is contraindicated, a CT scan may be warranted. CT scans are generally contraindicated in children and pregnant women due to radiation exposure. The contrast used in a CT scan is iodine-based, which may cause allergic reactions. This contrast is contraindicated in patients with iodine or shellfish allergies, as well as in patients with kidney problems. Therefore, BUN and creatinine levels are often needed before contrast administration.24
A CT scan is better at imaging blood and bone than an MRI, and is the test of choice for detecting trauma. An MRI with diffusion-weighted imaging is best at detecting acute strokes, as in an older patient with an internuclear ophthalmoplegia (INO).25 T2 and FLAIR MRI sequences are best at detecting white matter changes from multiple sclerosis, as in a younger patient with an INO from that disease process.26
Main Differential Diagnosis for Vertical Diplopia/Vertical Ocular Misalignment
Main Differential Diagnosis for Horizontal Diplopia/Horizontal Ocular Misalignment
|
Neuroimaging is critical in many cases of efferent visual system issues. A patient with a partial, painful or pupil-involved CN III palsy needs an emergent CTA to rule out aneurysm. In children or pregnant women, MRA would be preferable due to radiation. If an aneurysm is equivocal on CTA or MRA, then a digital subtraction angiography is the gold standard to rule out an aneurysm.27,28 With a painful Horner syndrome, MRA, CTA or angiogram is needed emergently to rule out a carotid dissection. With an isolated non-painful Horner syndrome, imaging of the brain, cervical spine (c-spine), lung, soft-tissue neck and orbits may be needed due to the long course of the sympathetic pathway. Contrast is preferred to rule out a mass.29
If you suspect thyroid eye disease, and are not very concerned about an alternate process, an orbital CT will assess for enlarged bellies of the muscles as well as any compression of the optic nerve. If you have a differential diagnosis that includes not only thyroid eye disease but also another condition, such as orbital mass, an MRI with contrast would be a better option. For myasthenia gravis, the imaging study that may be warranted is a CT of the chest to rule out thymoma, which occurs in up to 20% of patients with myasthenia gravis.30
Aside from structural abnormalities, other systemic causes of efferent visual system problems also need to be ruled out. This is mainly done with laboratory testing. For inflammatory conditions, order erythrocyte sedimentation rate (ESR) and C-reactive protein. A complete blood cell count (CBC), ESR, C-reactive protein and platelet count must be performed on patients over the age of 50 when considering the possibility of giant cell arteritis (GCA). Elevated ESR, C-reactive protein and platelet counts may be consistent with GCA, as may be a reduced hemoglobin level. Although classically thought of as a disease affecting the afferent visual system, GCA can cause diplopia and ocular misalignment.31
If you suspect thyroid eye disease, order not only thyroid function tests such as T3, T4 and TSH but also anti-thyroid antibodies (thyroperoxidase antibody and thyroglobulin antibody). Sometimes only the thyroid function tests are ordered, found to be normal, and the work-up ends there. It is possible to have thyroid eye disease with normal thyroid function tests. The anti-thyroid antibodies may be the only abnormal lab test in these cases.32
If you suspect myasthenia gravis, the initial work-up includes the acetylcholine receptor antibodies. There are three such antibodies: binding, blocking and modulating. These are more likely to be positive in generalized myasthenia gravis. There is only about a 50% chance that one of the tests will be positive in a patient with purely ocular myasthenia gravis.33 If all three acetylcholine receptor antibodies are negative, and myasthenia is still suspected, other possible lab tests to perform are the antibodies to muscle-specific kinase (MuSK) and striated muscle antibodies (StrAbs).34,35
Common Lab Tests for Diplopia
|
Another non-laboratory test for myasthenia gravis is a single fiber electromyogram (EMG). For ocular myasthenia gravis, this needs to be performed on the frontalis muscle or the orbicularis oculi muscle, not on the forearm, as is the more common version of the test.36
If the neuroimaging work-up and laboratory tests do not reveal a cause of the efferent visual system problem, ask yourself whether the work-up was complete and whether additional evaluation is warranted. Consult with neuro-ophthalmology and neurology as appropriate. But remember, sometimes an efferent visual system problem can signify a medical emergency. If you suspect an aneurysmal CN III palsy or a carotid dissection, do not wait for the next available appointment with the specialist; call them immediately or send the patient to the ER with information about his clinical findings and concerning diagnosis.
Put Your Knowledge and Experience to Work
When examining for neuro-ophthalmic disease processes, the most important tasks for the primary care optometrist are to fully assess the efferent visual system, determine if there is a concerning abnormality, assess the potential urgency of the situation, and determine when referral is indicated. Sometimes it may be difficult to determine whether the work-up is comprehensive enough or if the neuroimaging was done properly or read correctly. In those cases, referral to neuro-ophthalmology or neurology is warranted.
Do not underestimate your role in the care of a patient with a neuro-ophthalmic disease process––you have the important task of being the first individual to identify a problem that needs further evaluation, and determining how urgent or emergent that problem may be. You have the tools to make a proper diagnosis, and a difference!
Dr. Malloy is the director of the Neuro-Ophthalmic Disease Service at Pennsylvania College of Optometry, Salus University, and clinical assistant professor of neurology at Hahnemann/Drexel College of Medicine in Philadelphia.
References
- Malloy KA. Neuro-ophthalmic disease basics: Focus on the afferent visual system evaluation. Rev Optom. 2008 Jun;145(6):67-82.
- Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008 Dec;115(12):2282-5.
- Berger JR, Kovacs AG. Downbeat nystagmus with phenytoin. J Clin Neuroophthalmol. 1982 Sep;2(3):209-11.
- Vestergaard P. Smoking and thyroid disorders--a meta-analysis. Eur J Endocrinol. 2002 Feb;146(2):153-61.
- Fetter M, Haslwanter T, Bork M, Dichgans J. New insights into positional alcohol nystagmus using three-dimensional eye-movement analysis. Ann Neurol. 1999 Feb;45(2):216-23.
- Freedman KA, Brown SM. Topical apraclonidine in the diagnosis of suspected Horner syndrome. J Neuroophthalmol. 2005 Jun;25(2):83-5.
- Cooper-Knock J, Pepper I, Hodgson T, Sharrack B. Early diagnosis of Horner syndrome using topical apraclonidine. J Neuroophthalmol. 2011 Sep;31(3):214-6.
- Moster ML, Galiani D, Garfinkle W. False negative hydroxyamphetamine test in Horner syndrome caused by acute internal carotid artery dissection. J Neuroophthalmol. 2003 Mar;23(1):22-3.
- Patel RR, Adam R, Maldjian C, et al. Cervical carotid artery dissection: current review of diagnosis and treatment. Cardiol Rev. 2012 May-Jun;20(3):145-52.
- Antonio-Santos AA, Santo RN, Eggenberger ER. Pharmacological testing of anisocoria. Expert Opin Pharmacother. 2005 Oct;6(12):2007-13.
- Loewenfeld IE. The Argyll Robertson pupil 1869-1969. A critical survey of the literature. Surv Ophthalmol 1969;14:199Y299.
- Thompson HS, Kardon RH. The Argyll Robertson pupil. J Neuroophthalmol. 2006 Jun;26(2):134-8.
- Draper EM, Malloy KA. Progressive visual and hearing loss secondary to neurosyphilis. Optom Vis Sci. 2012 Nov;89(11):e65-71.
- Gold DR, Shin RK, Bhatt NP, Eggenberger ER. Aberrant regeneration of the third nerve (oculomotor synkinesis). Pract Neurol. 2012 Dec;12(6):390-1.
- Kelly-Sell M, Liu GT. “Tonic” but not “Adie” pupils. J Neuroophthalmol. 2011 Dec;31(4):393-5.
- Kawasaki A, Kardon RH. Disorders of the pupil. Ophthalmol Clin North Am. 2001 Mar;14(1):149-68.
- Morris OC, O'Day J. Fatigable ptosis and pseudoretraction caused by myasthenia gravis. Clin Experiment Ophthalmol. 2004 Jun;32(3):303-4.
- Fakiri MO, Tavy DL, Hama-Amin AD, Wirtz PW. Accuracy of the ice test in the diagnosis of myasthenia gravis in patients with ptosis. Muscle Nerve. 2013 Dec;48(6):902-4.
- Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009 Nov;116(11):2236-43.
- Metz HS. Forced duction, active force generation, and saccadic velocity tests. Int Ophthalmol Clin. 1976 Fall;16(3):47-73.
- Hernowo A, Eggenberger E. Skew deviation: clinical updates for ophthalmologists. Curr Opin Ophthalmol. 2014 Nov;25(6):485-7.
- Chen VM, Dagi LR. Ocular misalignment in Graves disease may mimic that of superior oblique palsy. J Neuroophthalmol. 2008 Dec;28(4):302-4.
- Canga A, Kislikova M, Martínez-Gálvez M, et al. Renal function, nephrogenic systemic fibrosis and other adverse reactions associated with gadolinium-based contrast media. Nefrologia. 2014;34(4):428-38.
- Diogo LP, Bahlis LF, Carvalhal GF. Computerized Tomography Contrast Induced Nephropathy (CIN) among adult inpatients. J Bras Nefrol. 2014 Dec;36(4):446-50.
- Chuang MT, Lin CC, Sung PS, et al. Diffusion-weighted imaging as an aid in the diagnosis of the etiology of medial longitudinal fasciculus syndrome. Surg Radiol Anat. 2014 Sep;36(7):675-80.
- McNulty JP, Lonergan R, Brennan PC, et al. Diagnostic efficacy of conventional MRI pulse sequences in the detection of lesions causing internuclear ophthalmoplegia in multiple sclerosis patients. Clin Neuro-radiol. 2014 Mar 6. [Epub ahead of print.]
- Lee AG, Hayman LA, Brazis PW. The evaluation of isolated third nerve palsy revisited: an update on the evolving role of magnetic resonance, computed tomography, and catheter angiography. Surv Ophthalmol 2002;47:137-157.
- Vaphiades MS, Cure J, Kline LB. Management of intracranial aneurysm causing a third cranial nerve palsy: MRA, CTA or DSA? Sem Ophthalmol 2008;23:143-150.
- Davagnanam I, Fraser CL, Miszkiel K, et al. Adult Horner's syndrome: a combined clinical, pharmacological, and imaging algorithm Eye (Lond). Mar 2013; 27(3): 291–298.
- Mao ZF, Mo XA, Qin C, et al. Incidence of thymoma in myasthenia gravis: a systematic review. J Clin Neurol. 2012 September; 8(3): 161-169.
- Davies GE, Shakir RA. Giant cell arteritis presenting as oculomotor nerve palsy with pupillary dilatation. Postgrad Med J. Apr 1994; 70(822): 298-299.
- Khoo TK, Bahn RS. Pathogenesis of Graves' ophthalmopathy: the role of autoantibodies. Thyroid. 2007 October; 17(10): 1013-18.
- Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscular Disorders. 2006;16(7):459–467.
- Selcen D, Fukuda T, Shen XM, Engel AG. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology. 2004 Jun 8;62(11):1945-50.
- Choi Decroos E, Hobson-Webb LD, Juel VC, et al. Do acetylcholine receptor and striated muscle antibodies predict the presence of thymoma in patients with myasthenia gravis? Muscle Nerve. 2014 Jan;49(1):30-4.
- Valls-Canals J, Povedano M, Montero J, Pradas J. Stimulated single-fiber EMG of the frontalis and orbicularis oculi muscles in ocular myasthenia gravis. Muscle Nerve. 2003 Oct;28(4):501-3.