Kids and Pharmaceuticals: How to Tailor Your Therapy
Treating infections in a pediatric population can be tricky. These tips and tricks can help.
By , and
Release Date: October 2017
Expiration Date: October 15, 2020
Goal Statement: Pediatric patients differ from adults in their pharmacokinetic responses to medications, and as a child grows, these differences evolve markedly, making treatment with pharmaceuticals particularly challenging. This article discusses the many ocular infections common in the pediatric population, as well as how to select the proper pharmaceuticals and calculate pediatric dosing.
Faculty/Editorial Board: Rachel A. Coulter, OD, MSEd; Julie A. Tyler, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 55140-OP. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Riviello and Michael Iannucci all have no relationships to disclose.
Pediatrics in the Optometric PracticeFollow the links below to read the other articles from our report on pediatrics in the optometric practice: Testing Children for Accommodative and Convergence Disorders |
Pediatric patients—a catch-all term that includes infants (birth to up to one year), toddlers (ages one to three), preschoolers (three to five), grade-schoolers (five to 12) and adolescents (12 to 18)—are not simply miniature adults; they differ in their pharmacokinetic responses to medications.1 Infants and toddlers, in particular, have the greatest differences from adults in drug absorption, distribution, metabolism and excretion, and as a child grows and matures, these differences evolve markedly.2
This article discusses managing eye infections in children and highlights how optometrists can tailor the therapy to match the needs of each pediatric patient.
Prescribing Challenges
For clinicians seeking information on appropriate prescribing for specific medications, the first resource should be the Pediatric Use section found in the manufacturer’s drug insert.3 Although randomized clinical trials to assess how infants and children respond to pharmaceuticals continue to lag behind trials assessing adults, drug inserts are more likely than in the past to include pertinent information, including the age for which the drug is approved, any associated side effects and outcomes.4,5
 |
| Preseptal cellulitis, here in two different patients, can be caused by sinusitis, chalazia, hordolea, dacryocystitis, trauma or insect bites. Click image to enlarge. |
When pediatric prescribing information is not available, the Pediatric Use section will state, “Safety and effectiveness in children have not been established.”3,6 In these circumstances, off-label use may be necessary, which can be a significant problem in ocular care. In fact, research shows eye drops and dermatologic preparations are the most likely to be prescribed off-label in the primary health care setting.2 When practitioners discuss treatment options with the parent or guardian, they should include whether or not the drug has been approved for pediatric use, and document the decision-making process and the parent’s/guardian’s consent in the medical record.
When prescribing off label, optometrists should first check the package insert and FDA website for warnings or contraindications, as some commonly used drugs have serious side effects specific to pediatric patients. For example, doxycycline and other tetracycline analogs can cause dental enamel abnormalities in some children younger than eight.7 Likewise, over-the-counter cough and cold medications are associated with an increased risk of respiratory depression, seizures and arrhythmias in children younger than four.8
Determining Dosage
The best source for this task is the Dosage and Administration section in the package insert. When pediatric dosage information is not provided for topical ophthalmic drugs, researchers suggest clinicians use half the adult dose for children younger than two and two-thirds the adult dose for children ages two to three.9,10 Often, clinicians decrease the frequency of dosing of ophthalmic preparations, as drops are difficult to “split.”
 |
| Pediatric patients who present with localized swelling, pain and redness may have a hordeolum, as seen here. Photo: Christine W. Sindt, OD |
Despite differences in pharmacokinetics and pharmacodynamics between children and adults, oral medications in the pediatric population are still adjusted according to body weight (mg/kg/day) or, in the case of chemotherapeutic drugs, according to body surface area (mg/m2).11 To establish the patient’s weight, optometrists should weigh the patient in office and not rely on reported weight. Optometrists considering oral medications for ocular conditions in a pediatric patient—such as amoxicillin suspension for preseptal cellulitis—should take a four-step approach: (1) convert pounds to kg (1kg = 2.2lb), (2) calculate the dose in mg, (3) divide the dose by frequency and when needed for oral solutions/suspensions and (4) convert the mg/dose to mL.
Common Infections
Pediatric patients with an infectious condition might be unable or unlikely to report their symptoms. Consequently, diagnosis is often made based upon findings and the history reported by the parent or caregiver, including the condition’s duration, laterality, the presence of pain and photophobia, eye discharge and any history of trauma.12-14
Optometrists can choose from a wide variety of antibiotics when managing bacterial eye infections in pediatric patients. Clinicians must consider the type of infection, severity and the age of the child to determine the best choice. Other factors that influence medication selection include the availability of the pharmaceutical in ointment or solution form, length of treatment, number of doses per day and the cost.15
Here are some prescribing considerations for several of the more common infections found in the pediatric population:
Blepharitis may be associated with bacterial infections with vessel telangiectasias and hard, fibrinous crusts and scales with occasional misdirected or missing lashes.16 It most commonly presents in children between ages six and 10.12 Treatment starts with lid hygiene, but topical and oral antibiotics are added when necessary to resolve severe and chronic cases.17-19 Lid scrubs may include diluted baby shampoo applied to the lid margins or commercial wipes, including Systane lid wipes (Alcon) or Ocusoft lid scrubs, which are available in a “baby” formulation.18 As Staphylococcus overgrowth is a major causative factor of lid inflammation, crusting and flaking, antibiotics particularly effective in reducing these bacteria are good options.17,19 A good choice is ophthalmic erythromycin ointment applied one to two times a day for two weeks and discontinued when the condition improves. Because ointments cause blurry vision, applying them close to bedtime may improve compliance.
| Beyond the Prescription Pad
|
While AzaSite (azithromycin ophthalmic solution 1%, Akorn) is FDA approved for bacterial conjunctivitis, it has known benefits against lid disease as well. In addition to targeting Staphylococcus, it improves meibomian gland function by enhancing the immunomodulatory response.19,20 AzaSite is typically dosed BID for two days OU and then changed to once a day for the next five days.19,20 It has a good safety profile and is FDA-approved for children 12 months and older. Unfortunately, it is difficult to find in pharmacies and is often expensive.
In severe blepharitis, a topical antibiotic/steroid combination may eliminate inflammation and bacterial overgrowth.21,22 However, side effects of corticosteroids include elevated intraocular pressure (IOP) and cataract formation, and the risk increases with chronic use.17 In addition, herpes simplex virus (HSV) may be the culprit on rare occasions, and steroid treatment may worsen the condition and negatively impact the cornea.
Roughly 5% of chronic pediatric blepharitis cases progress to corneal involvement in the form of punctate erosions, punctate keratitis, phlyctenules, marginal keratitis or ulceration; in these cases, more aggressive management is required, which may include oral antibiotics or a topical fluoroquinolone with broad spectrum coverage such as besifloxacin.22
Topical antibiotic/steroid combinations in ointment form, including Tobradex (tobramycin and dexamethasone ophthalmic suspension, Alcon) or neomycin/polymyxin B/dexamethasone, may be easier to use in pediatric patients than an eye drop.2 Clinicians should limit steroid use, and carefully consider the dosing frequency and risks/benefits prior use in children.
When an oral antibiotic is necessary for severe or chronic lid disease, oral azithromycin is a good choice, as it has both anti-inflammatory and antibacterial properties, and the oral formulation is approved for children six months of age and older.20
Hordeola, caused by infections of the eyelid glands, can cause localized swelling, pain and redness. Usually caused by Staphylococcus aureus, hordeola may initially be treated by applying a warm compress to the eye for 10 to 15 minutes, four times a day.14 To help children keep the warm compress on the lid/face, the parent might challenge the child to “freeze” it in place using music as a timer. The “game” is to not remove it until the end of the song (or songs). While topical antibiotics generally are not used to treat hordeola, they may be necessary to prevent the spread of infection to other structures when drainage/expression is significant. On occasion, internal hordeola may progress and result in a secondary preseptal cellulitis that requires an oral antibiotic.
Conjunctivitis is a common eye disease, responsible for approximately 1% of all primary health care visits.23 While infectious conjunctivitis may be bacterial or viral, most conjunctivitis in children is bacterial.24 Bacterial conjunctivitis is typically self-limiting, resolving in one to two weeks in more than half of all cases.23 Antibiotic treatment hastens resolution, decreases symptoms and reduces the risk of complications and disease transmission.25-27
The causative organisms of bacterial conjunctivitis in pediatrics are usually Staphylococcus aureus, Staphylococcus epidermis, Streptococcus pneumoniae, Moraxella catarrhalis, Pseudomonas and Haemophilus.21 The most marked symptom is a sticky mucopurulent discharge that coats the eyelashes and causes the eyelids to stick together.
For milder presentations, the initial treatment choice is often a broad-spectrum antibiotic effective against both gram-negative and gram-positive organisms.14 The most common treatment options include a combination of polymyxin B sulfate and trimethoprim sulfate ophthalmic solution, polymyxin B/bacitracin ophthalmic ointment and erythromycin 0.5%.14 Clinicians may also use topical aminoglycosides, including gentamicin 0.3% and tobramycin 0.3% drops or ointment. However, aminoglycosides are not as effective against Staphylococcus and are associated with a corneal epithelial toxicity reaction after several days of use.14 For infants, preschoolers and those who struggle with drop instillation, an ointment preparation may be helpful. Erythromycin is FDA-approved for use in newborns, while tobramycin is approved for use in children as young as two months of age.
Clinicians have several treatment options for more moderate presentations of bacterial conjunctivitis. AzaSite is effective against acute bacterial conjunctivitis, and research shows it is superior to tobramycin.28 It is dosed one drop in the affected eye(s) twice daily, eight to 12 hours apart for the first two days and then once daily for the next five days.29 This drop’s viscous vehicle enhances contact with the eye and enables a caretaker to gently run “excess” drop along the lid margins to provide additional treatment to any infection of the eyelid.
| Avoid Common Dosing Errors 1. Mitchell AL. Challenges in pediatric pharmacotherapy: Minimizing medication errors. Medscape. May 21, 2001. |
A fluoroquinolone is another option for bacterial conjunctivitis, and an increasing number are FDA approved for use in children older than 12 months. Older generations include ciprofloxacin, ofloxacin, moxifloxacin and gatifloxacin. Ciprofloxacin 0.3% is also available in an ointment form and is approved for use in children two years and older. Dosing should be frequent with severe cases, particularly during the first days, and the fluoroquinolone choice will determine the exact frequency. For example, ciprofloxacin 0.3% solution should be dosed every two hours while awake for the first two days and then every four hours for five days; moxifloxacin 0.5% solution is dosed two or three times per day for a week, depending on the solution vehicle.
A topical fluoroquinolone may also be a good choice for pediatric patients who are contact lens wearers because of the association between Pseudomonas infection and contact lens wear.14 Children who have conjunctivitis and are contact lens wearers should immediately discontinue contact lens wear, discard their current contact lens case, if applicable, and not return to contact lens wear until the condition is resolved and no indication of discharge exists.
For severe cases of bacterial conjunctivitis, clinicians should consider a newer generation of fluoroquinolone such as Besivance (besifloxacin 0.6% ophthalmic suspension, Bausch + Lomb). Research shows this broad-spectrum antibiotic is safe and effective for children one year and older.30 Studies also show this chlorofluoroquinolone is effective even against multi-drug resistant Staphylococci. Besivance is prescribed TID for seven days for bacterial conjunctivitis.26
Optometrists should see patients undergoing treatment for conjunctivitis every two to three days until signs and symptoms resolve. Bacterial conjunctivitis is quite contagious and is often transmitted through hand-to-eye contact.31 Although cultures are expensive and usually are unnecessary, clinicians can consider gram stain, culture and drug sensitivity to clarify diagnosis and treatment in cases where a child is not improving or there is a concern about resistance.28 Antibiotic resistance is not uncommon in pediatric conjunctivitis cases, particularly when the child has co-occurring otitis media.32 Conjunctival discharge may contribute to the spread of antibiotic resistant bacteria.
Patient education regarding ocular hygiene is key.33 Clinicians should remind parents and caregivers of common practices to reduce the transmission of bacteria:
- Avoid touching the eyes or eye area.
- Use disposable tissues and wipes instead of towels.
- Wash hands immediately after touching the eyes or eye area.
- Don’t share towels, washcloths, dishes, cups or eating utensils.
- Play with hard surface toys that can be thoroughly cleaned.
Research shows these hygiene practices can reduce daycare absenteeism among children age three and younger.34
Patients may initially present to their primary care physician, which means that a non-eye care provider may make the initial diagnosis and initiate treatment. When a child has a persistently red eye, but no discharge or fever, optometrists should review the chosen treatment with the primary care physician. Gentamicin and neomycin, for example, may be associated with toxic reactions and red eyes that persist beyond the bacterial infection.14
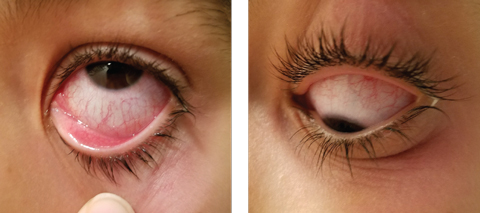 |
| This child has bacterial conjunctivitis in the left eye. On upgaze, note the injection as well as discharge around caruncle nasal. Papillae are visible on the lower lid. On downgaze, you can see Injection of the bulbar conjunctiva and noticeable redness around the adnexa. Click image to enlarge. Photo: Alexandra Espejo, OD |
Neonatal bacterial conjunctivitis, though not common in optometric practices, may be linked to Neisseria gonorrhoeae or other sexually transmitted diseases, which neonates may contract during the birth process. Neisseria gonorrhoeae results in a hyperacute bacterial conjunctivitis that develops quickly after birth and rapidly progresses. It is marked by copious, purulent discharge that quickly reforms after being wiped away. The condition requires medical comanagement with a neonatal specialist, ophthalmologist or pediatrician, in cases of hyperacute conjunctivitis or when systemic association is suspected. Aggressive treatment is required, as corneal perforation is a risk with Neisseria gonorrhoeae infection.14 Treatment measures include irrigation of the eye with saline, systemic ceftriaxone (which may be IV in infants) and topical ciprofloxacin.14
A recent study compared research on the use of two different topical fluoroquinolones (besifloxacin 0.6% vs. gatifloxacin 0.3%) in 33 neonatal subjects with bacterial conjunctivitis. Clinically, both medications were safe, well tolerated and demonstrated high rates of clinical resolution with TID dosing for one week.35
Viral conjunctivitis is usually bilateral—with symptoms starting in one eye and progressing to the second. Infected patients present with red, watery eyes and sometimes small hemorrhages. In children with a developed immune response, clinicians may see pre-auricular nodes. The most common form of viral conjunctivitis is caused by adenovirus, although HSV and varicella zoster can also be etiologies.23 In some adenoviral conjunctivitis sub-types, a delayed corneal response of subepithelial infiltrates may occur; therefore, clinicians should emphasize patient and parent education regarding expected outcomes and the importance of follow-up.
Viral conjunctivitis is a self-limiting condition and symptoms usually decrease within the first week. Children should be kept out of school or daycare for several days because the condition is highly contagious.31 Currently, no ophthalmic agents are approved for adenoviral infections. Moreover, use of an antibiotic does not hasten resolution and is not indicated, unless there is super-infection by bacteria. Clinicians should instruct parents and patients on general preventative hygiene measures and palliative measures such as cool compresses and artificial tears.
Microbial keratitis (MK) is relatively rare in the pediatric patient, with notable exceptions for children who experience ocular trauma or wear contact lenses.36-38 Additional risk factors for MK include decreased immune system due to early development or an underlying systemic condition, chronic dacryocystitis, canaliculitis or blepharitis, or ocular trauma with a secondary opportunistic infection. Most aspects of a pediatric keratitis presentation will be similar to an adult presentation, including the presence of an epithelial defect—generally overlying a stromal infiltrate—with associated corneal edema and symptoms of blurred vision, pain, tearing and light sensitivity. In children, the most common bacteria responsible for keratitis varies by associated predisposing factor—with gram-positive microorganisms the main agents of infection in children unless associated with contact lens wear, in which case Pseudomonas aeuriginosa is most notable.36-38 Other causative agents of keratitis include viral and fungal infections.
In all cases of keratitis, the goal is to remove the causative/contributing agents, provide anti-infective agents when available and initiate anti-inflammatory support. Removing insulting agents, such as contact lenses, is a key first step. Upon diagnosis, in-office management with a sterile saline lavage, followed by home lavage with saline, artificial tears or both can provide mechanical support and removal of infectious agents and toxic debris. Palliative treatment with cool compresses and possible cycloplegic agents is also helpful.
The common agents used to treat a presumed bacterial keratitis are fluoroquinolones, especially with history of soft contact lens wear. However, the treatment regimens are often off-label, even in adult populations, as only earlier generations have FDA approval and dosing regimens. Fortunately, several of these medications are approved for young children. Clinicians should also consider overnight coverage, and possibly daytime treatment in very young children, with an antibiotic ointment. Patients should be monitored daily until resolution, and if a specific antibiotic regimen is working for a child, clinicians should not alter it until corneal epithelial improvement is marked.
Viral keratitis with a primary corneal defect is most likely due to HSV. Patients may present with conjunctival injection, follicles and corneal staining with rose bengal of a classic dendrite or, early on, as discrete punctate defects. The two primary FDA-approved topical medications for HSV epithelial keratitis include trifluridine 1% drops—generally dosed up to nine times per day and with noted corneal toxicity over time—and Zirgan (ganciclovir ophthalmic gel 0.15%, Bausch + Lomb).39 Zirgan has lower corneal toxicity, less frequent applications and a gel formulation that may be preferable in some pediatric populations.39 Research has yet to establish its safety and efficacy in patients younger than age two.
Also, while corneal debridement may be considered in adults with HSV epithelial keratitis, it is not recommended in the pediatric population because the ability to achieve a precise debridement without damaging Bowman’s layer in children is a significant challenge.
Fungal keratitis, in general, is difficult to manage because few effective anti-fungal agents exist and the condition is quite invasive.40 If fungal keratitis is suspected based on history, lack of response to antibiotics, or development of a thickened, feathery, poorly defined corneal lesion, clinicians should consider comanagement with an ophthalmologist. The challenges of managing a fungal infection are amplified in pediatrics due to a dearth of information on anti-fungal agents in this population and, in some cases, a less well-developed natural immune response.
Preseptal cellulitis is an infection of the eyelid and periorbital tissue that is anterior to the orbital septum. Common signs are lid swelling and redness, often with fever and discharge.41 The orbital septum is a thin, fibrous membrane that serves as a barrier to prevent deeper infection manifesting as orbital cellulitis that requires more aggressive treatment. When the infection involves tissue posterior to the orbital septum, the condition is diagnosed as orbital cellulitis. Signs and symptoms of orbital cellulitis include proptosis, decreased ocular motility, severe pain of the orbit, afferent pupillary defect, optic nerve head edema and decreased visual acuity. Differentiating orbital cellulitis from preseptal cellulitis is important, as orbital cellulitis is potentially a vision- or life-threatening condition.
Causes of preseptal cellulitis include sinusitis, chalazia, hordolea, dacryocystitis, trauma and insect bites.41 Treatment requires oral antibiotics. Amoxicillin and Augmentin (amoxicillin/clavulanic acid, GlaxoSmithKline) are good options for children without a penicillin allergy.42 Augmentin, which can be prescribed in several formulations and flavors, is prescribed at levels of 20mg/kg/day to 40mg/kg/day for no more than 10 days. Amoxicillin and Augmentin are approved for use in neonates and infants.43
Clinicians can consider prescribing trimethoprim/sulfamethoxazole when patients present with infections resistant to other antibiotics. Septra (trimethoprim/sulfamethoxazole, Monarch Pharmaceuticals) and Bactrim (trimethoprim/ sulfamethoxazole, Roche) are contraindicated for children younger than two months of age, as well as for patients with sickle cell disease or sulfa allergies.42 Clinicians should note that use of either of these medications is associated with a higher risk of Stevens-Johnson syndrome.42 Dosage depends on the type and severity of the infection.
Because pediatric eye infections commonly present in primary care optometric practices, clinicians need to be aware of differences in selecting pharmaceuticals, dosing, patient education and treatment measures. Armed with the right information about prescribing for children, optometrists can create a pediatric following in their practice.
Dr. Coulter is a professor at Nova Southeastern University. The focus of her clinical work is pediatric and special needs patients.
Dr. Tyler is an associate professor and module chief at Nova Southeastern University. She serves as instructor of record for the course, Ocular Disease of the Anterior Segment.
1. American Academy of Pediatrics. Ages and Stages. 2017. www.healthychildren.org/English/ages-stages/Pages/default.aspx. Accessed July 31, 2017 |
