10th Annual Cornea ReportFrom advice on diagnosing and treating infections to managing trauma and limiting recurrent erosion, the April 2023 issue is full of clinical pearls to help you up your corneal management skills. Check out the other articles featured in this issue:
|
Despite being one of the more prevalent corneal conditions we encounter, keratoconus still holds many mysteries for clinicians and patients alike. During one festive evening at the 2023 Global Specialty Lens Symposium in Las Vegas, various experts were asked, “What surprises you about keratoconus?” Below, you will find their responses followed by a literature review that lends context and background information to their experiences and insights.
Responses from the experts have been edited for brevity and clarity. Also note that their replies were meant to be off-the-cuff and thought-provoking rather than carefully considered, evidence-based commentaries, so there is an element of levity to some of the thoughts shared here.
Diagnosis and Initial Encounter
The consistent theme emerging during our discussion with experts was the critical importance of early identification of keratoconus patients and suspects, given the lifelong impact of the condition.
“The number of times keratoconus has not been detected.”—Tom Quinn, OD
In a recent systematic review and meta-analysis, keratoconus prevalence was reported to occur in around 1.38 per 1000 people as an average across the globe.1 This differs from an oft-cited study done by Godefrooij et al., which reports that the prevalence is much higher and measured at 1:375 in their study done in the Netherlands.2 While prevalence varies depending on the population analyzed in each study, the simple truth remains: keratoconus cannot be reported if it is not detected.
The diagnosis can elude doctors who don’t perform proper imaging and can be misdiagnosed as anisometropic or refractive amblyopia, high astigmatism, vernal keratoconjunctivitis and more. Keratoconus onset has traditionally been reported to be towards the middle of the second decade of life, but self-reported onset for patients is often not until a few years later.3,4 For patients diagnosed after that, there are many years of missed opportunities for treatment.
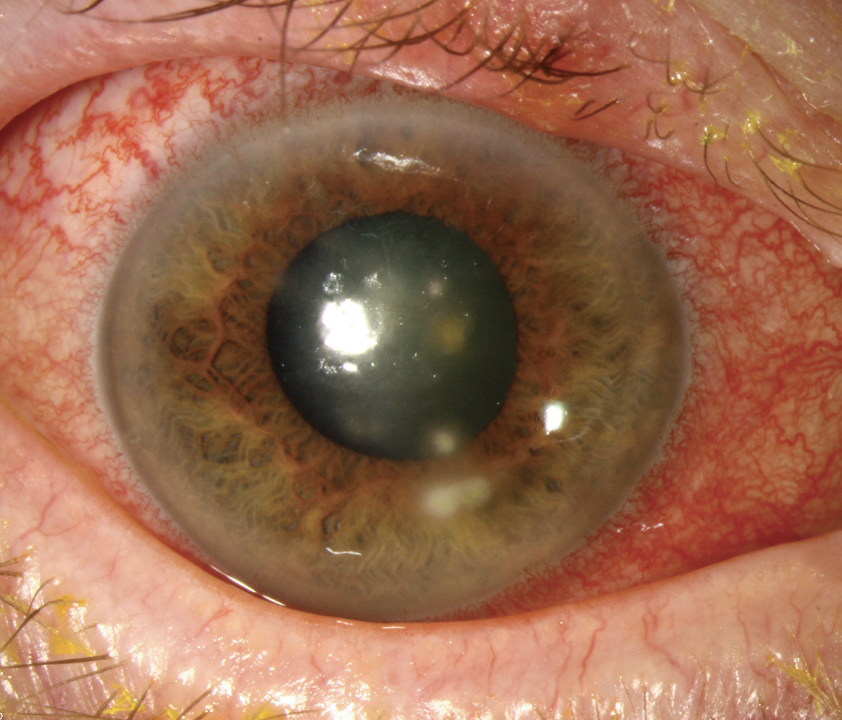
 |
|
Dramatic presentations like this may be unambiguous cases of keratoconus, but experts agree that our emphasis should be on gaining a more nuanced understanding of the condition’s subtle early signs to allow for swift intervention and a better long-term prognosis. Click image to enlarge. |
While the diagnosis can be simple when patients present with more advanced cases and evidence of clinical signs, it can be more difficult in very early or forme fruste keratoconus. Detecting these more difficult cases that do not have obvious corneal slit lamp findings requires additional testing, including corneal topography, and potentially corneal hysteresis, corneal tomography and aberrometry. Through corneal biomechanics testing, key markers to watch out for include deflection amplitude of highest concavity and stiffness parameter at first applanation.5
With corneal tomography, clinicians should direct their attention to the front elevation thickness at the cornea’s thinnest location as an indicator for keratoconus.5 Patients with keratoconus, and other non-inflammatory thinning disorders such as Pellucid’s marginal degeneration, also have a higher incidence of higher-order aberrations, especially coma-like aberrations, which can be detected with an aberrometer.6
“It’s possible that some cases are being missed and masked by the surge in practitioners launching their pediatric myopes into orthokeratology lenses without ruling out early ectatic disease with a simple Pentacam image. Pediatric optometrists and ophthalmologists should be the front line of early keratoconus detection—but they are not. In an ideal world, Pentacam imaging would be a mandatory part of a comprehensive annual pediatric eye exam.”—Andrew Morgenstern, OD
Corneal topography and tomography scans remain a standardized method of diagnosing keratoconus. However, when eyecare practitioners do not have access to these devices, corneal ectatic disease can be misdiagnosed in the earlier stages. While taking and having pediatric patients sit for imaging can be difficult, the lack of standardized care to screen for keratoconus can prove to be detrimental to patients who fall through the cracks. Even though the age of onset is postulated to be around 15 years of age, kids can start to manifest early signs even before that. These early-onset patients tend to be battling more aggressive forms of the disease and need treatment as soon as possible.
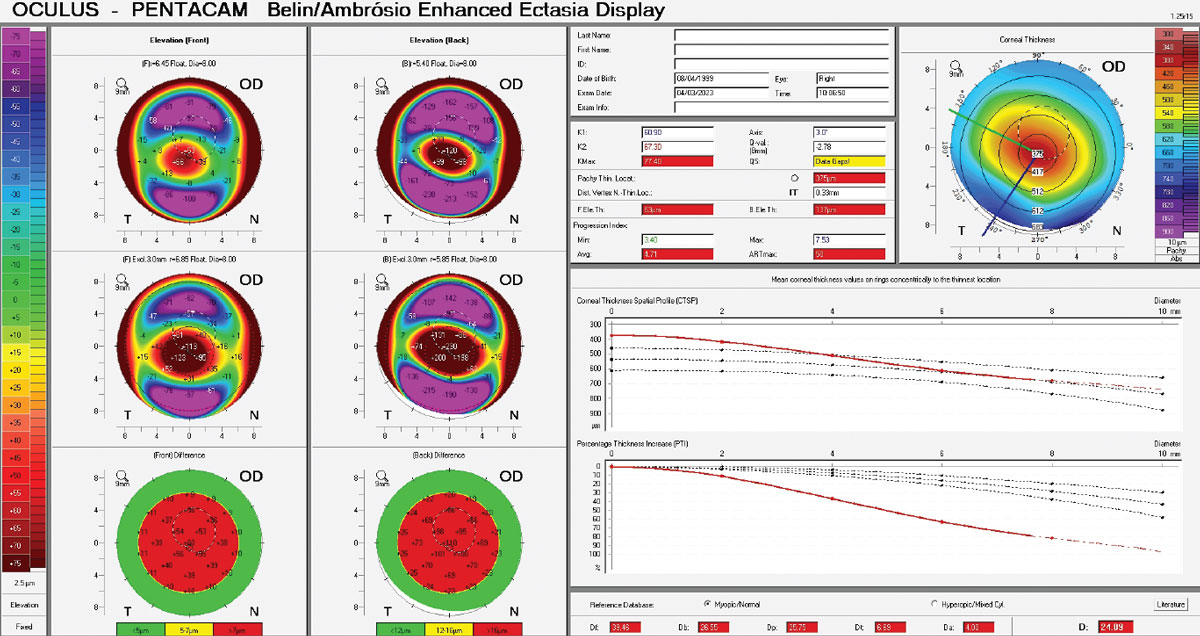
For those practicing in settings where corneal tomography scans are accessible, there are a few key areas to evaluate closely.7 In borderline diagnosis cases, your suspicion for keratoconus should be elevated when seeing Belin/Ambrósio values ≥1.54, 5th-order vertical coma aberration of the front cornea ≥0.023, Index of Surface Variance values ≥22 and Index of Vertical Asymmetry values ≥0.14.7
See Table 1 for generally accepted diagnostic parameters of definite keratoconus.
“It always starts on the back of the cornea. If you only did topography, you messed up.”—Buddy Russell, FCLSA
One of the earliest signs of keratoconus is steepening of the posterior elevation, which can be adequately measured with corneal tomography, but not topography, scans. While topography can be adequate to initially detect mild/severe keratoconus, its use alone will not be enough to confirm corneal ectasia in early cases.
For early and forme fruste keratoconus eyes, a cut-off level between 20.0µm and 26.5µm of posterior elevation can differentiate definite keratoconus when compared to normal eyes.8 In order to diagnose these patients with pre-topographical keratoconus, additional data that can be gained from corneal tomography scans are critical. For eyecare practitioners who do not have access to corneal tomography, there is always the opportunity to refer these patients to other doctors who do, so that they can perform the scan.
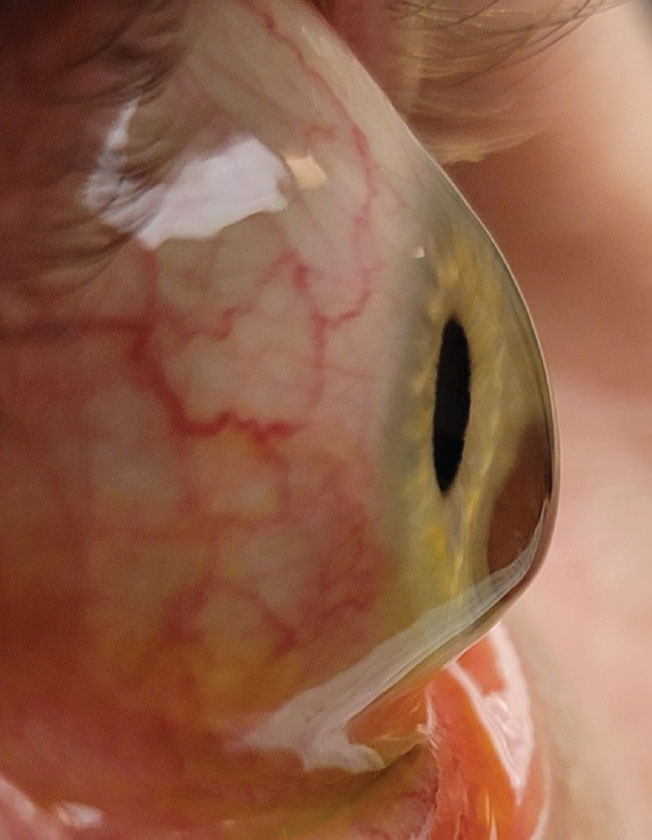
 |
|
Corneal tomographic data from the Belin/Ambrósio report of a 24-year-old keratoconus patient. The pacymetry progression index calculates the change in corneal thickness from the thinnest point to the periphery. A rapid change in thickness indicates ectasia. Click image to enlarge. |
“HOAs suck! We need good aberrometers to measure them.”—Tiffany Andrzejewski, OD
The introduction of wavefront-sensing aberrometers into optometric and ophthalmic clinical practice has greatly helped us quantify the cornea’s optical/refractive performance. Various studies have reported an increase in the number of higher-order aberrations (HOAs) that patients with keratoconus experience, particular coma induced by superior-inferior asymmetry of keratoconic corneas; these can significantly impact visual impairment.9 Several researchers have explored using wavefront sensing to detect early and forme fruste keratoconus—in other words, pre-topographic keratoconus—where increased corneal total HOAs and corneal coma can manifest themselves.9-11
Combining videokeratography and wavefront analysis can improve the specificity and sensitivity for early detection of the disease.12 The magnitude and type of HOA changes as keratoconus progresses, but also potentially after procedures such as CXL are performed. A study done by Greenstein et al. examined the effect of crosslinking and Intacs on HOAs and found that total HOAs and vertical and horizontal coma of the anterior cornea decreased, while spherical anterior corneal HOAs increased and trefoil was not affected.13 This becomes important when monitoring patients for progression in their disease process, as well as for designing wavefront-guided contact lenses for patients pre- and post-surgery.
“I’m surprised by the number of keratoconus patients who are happy with the vision they’re entering with.”—Jonathan King, OD
While there is not a lot of literature on uncorrected visual acuity in keratoconus patients who first present to clinic, most practitioners can relate to the surprise felt when examining a patient for first-time glasses or contact lenses who has never worn any correction before. In one study done in Tel Aviv, investigators found entering uncorrected acuity for patients with mild/moderate keratoconus and no corneal scarring to be 1.10±0.68 logMAR, which is about 20/250 for its Snellen equivalent.14
Depending on the asymmetric nature of the keratoconus as well, patients who are essentially functioning monocularly may not come in for visual distortion as early as patients whose keratoconus is more symmetric. No matter the reason or timeframe of the patient presenting in your chair, herein lies an opportunity to offer them a chance to achieve functional vision. The early referral for corneal collagen crosslinking (CXL) not only could help slow or halt disease progression but could also even help patients stay successful in spectacle lens wear for adequate visual correction.
 |
| Click image to enlarge. |
Management and Prognosis
Clearly, the introduction of CXL has been a game-changer for keratoconus, and the recent renaissance in scleral lens fitting has given optometrists even more capabilities to provide the best possible vision.
“When you’re managing keratoconus, you’re not just managing their corneal ectasia—you’re always taking care of a whole person. We need to spend more time correlating keratoconus with systemic diseases the patients may have.”—Jennifer Harthan, OD
While we typically use clinical signs and clinical tests to diagnose keratoconus, there may be additional comorbidities the patient is battling that are either early clues or diseases that worsen the disease.
For keratoconus suspects, asking pertinent questions about a history of eczema, asthma, allergy, and eye rubbing should be a part of the entering case history.1 In those already diagnosed with keratoconus, the aforementioned conditions still need to be managed, as well as sleep apnea, connective tissue disorders, inflammatory bowel disease, allergic rhinitis, diabetes and atopy.15
Eyecare practitioners have the ability to communicate with other healthcare providers to make recommendations and follow up on our guidance so that our keratoconic patients have an adequate team of experts to support them for all aspects of their well-being.
“Crosslinking has been proven effective for decades and yet not enough patients today do it. Get CXL first and then we’ll talk about contact lens fitting.”—Shalu Pal, OD
Since its introduction internationally in the late 1990s and its US FDA approval in 2016, CXL has revolutionized the way practitioners manage and treat keratoconus. The potential reduction in the need for penetrating keratoplasty, the reduction in disease progression and the biochemical stiffening of the ectatic cornea are all made possible by the formation of strong covalent bonds from riboflavin drops and ultraviolet A light interactions with stromal collagen fibrils.16
Currently, the standard CXL procedure is still the Dresden or “epithelium off” protocol.17 Epithelium-on procedures and other variants are being studied to try to reduce the incidence of infection and improve comfort for patients post-procedure to allow more patients to be able to undergo the procedure. However, current research has shown the Dresden protocol is still the most effective in mitigating disease progression.18
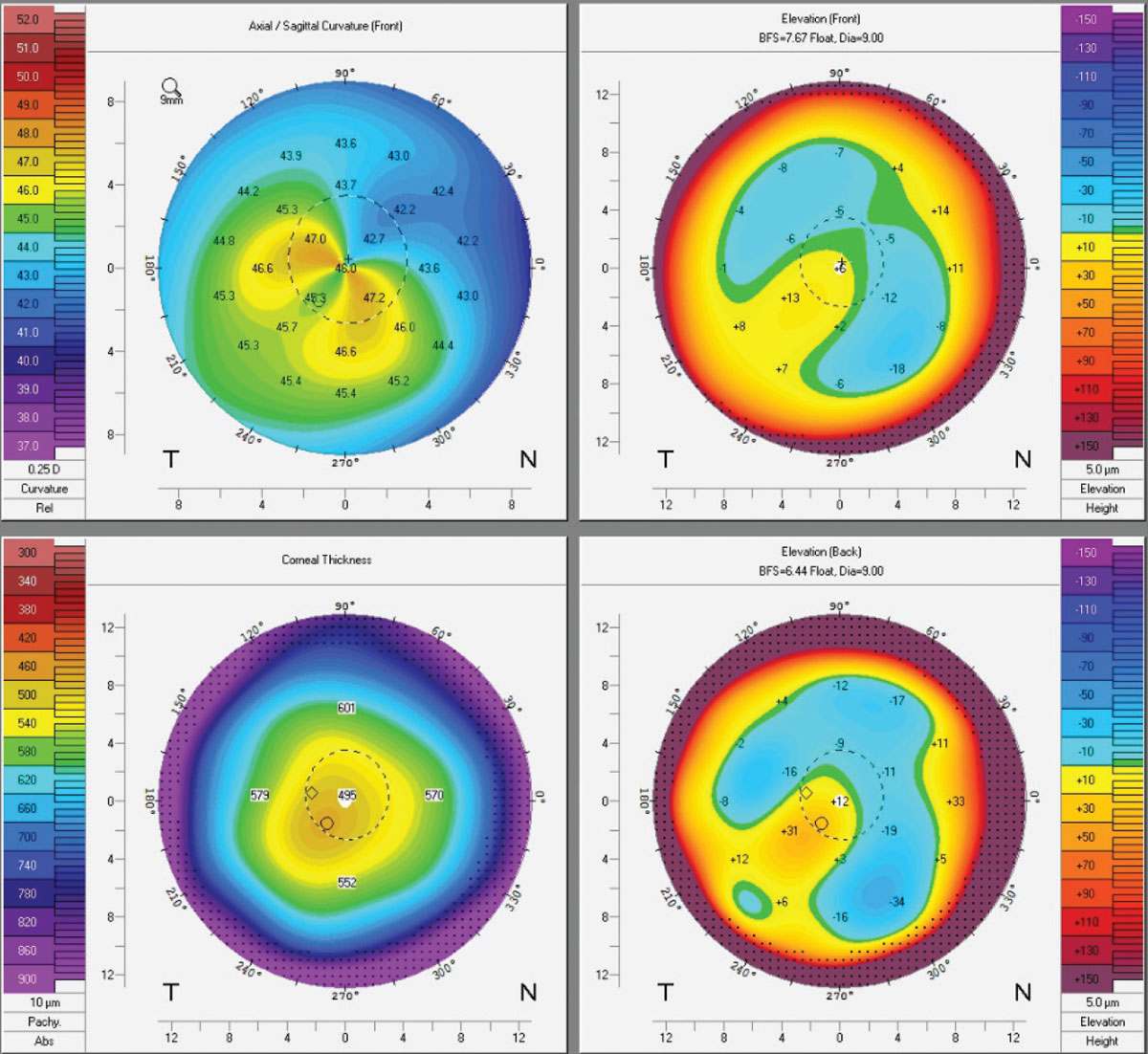 |
|
Topographies of an early keratoconus patient post-CXL. Crosslinking binds collagen fibers to adjacent ones using riboflavin drops and UV light. While some Kmax flattening occurs, patients should be told that CXL is done to reduce progression, not improve vision. Click image to enlarge. |
The most common potential adverse event from CXL is corneal haze, while the least common are microbial keratitis and stromal melt. Depending on severity, all complications may affect postoperative visual acuity.19
The ideal timing of referral can be tricky. While practitioners may wait to refer adult patients for CXL until progression is noted, pediatric keratoconus usually indicates a more aggressive phenotype that requires earlier intervention. Timing can also depend on visual demands and whether or not the patient is able to wait until after CXL to be able to achieve adequate acuity for their activities of daily living.
Anterior or posterior corneal surface steepening and a change in corneal thickness or rate of thickness change from the thinnest point to the periphery are all considered signs of progression. The presence of two out of three of these is considered progression, according to some; however, there is no uniformly accepted definition of the phenomenon.20 Ultimately, corneal crosslinking is a relatively safe and effective intervention to reduce and potentially halt progression for the majority of keratoconic eyes.21
“I am surprised by how few solutions these patients have been given and how hard they struggle.”—Daniel Neal, PhD
Visual impairment in general can significantly impact quality of life and is considered a common cause of psychological distress.22 A general decline in acuity also has a positive association with depressive symptoms.23-24 In a study done to compare the frequency and intensity of depression in keratoconic patients with their non-keratoconic age- and gender-matched counterparts, the former experienced more depression, and at higher intensity, than healthy control subjects.25 It may be postulated that it is actually the subjective visual deterioration as opposed to lower best-corrected visual acuity that is actually correlated with developing depression.23-24
There are multiple ways to measure depression through screening questionnaires such as the following:26-32
- Hospital Anxiety and Depression Scale
- Hamilton Rating Scale for Depression
- Geriatric Depression Scale
- Zung Depression Inventory-Self Rating Depression Scale (Zung SDS)
- 36-item Short Form Health Survey
- 9-item Patient Health Questionnaire (PHQ-9)
- Beck Depression Inventory
The PHQ-9 and Zung SDS are particularly useful questionnaires that could potentially be incorporated into practice, since they are strongly and positively intercorrelated with keratoconic patients.25
“I wish both doctors and patients would not believe and persist in suggesting that a diagnosis of keratoconus will either lead to blindness or corneal transplants.”—Barry Eiden, OD
While keratoconus is a relatively rare condition, it generally has an onset during young adulthood, with 94% of patients diagnosed between the ages of 12 and 39, and can be an emotional and devastating diagnosis.33 Many patients mistakenly believe they will go blind from keratoconus and do not adequately understand the potential outcomes of the condition.
According to a 2005 study, the general population rates fear of blindness second only to fear of cancer and AIDS (a more consequential diagnosis at the time, prior to the introduction of lifesaving therapies), and therefore keratoconic patients may develop irrational fear of incipient blindness if they are not properly informed about their visual prognosis.34
Especially with the availability of CXL and scleral lenses, more and more patients are now able to slow or even halt the progression of keratoconus and completely avoid the need for corneal transplantation.35 It is therefore imperative to carefully monitor these patients for progression and offer them the appropriate protocols for CXL where indicated.36 Patients diagnosed at younger ages (less than 17 years old) and with a steeper Kmax (greater than 55D) at initial presentation are at increased risk of progression and should be monitored more closely.36
Additionally, there are many different contact lens modalities available to the eyecare practitioner to correct keratoconus, such as custom soft, gas permeable, hybrid and scleral lenses. The latter can now provide visual correction for even the steepest of corneas.
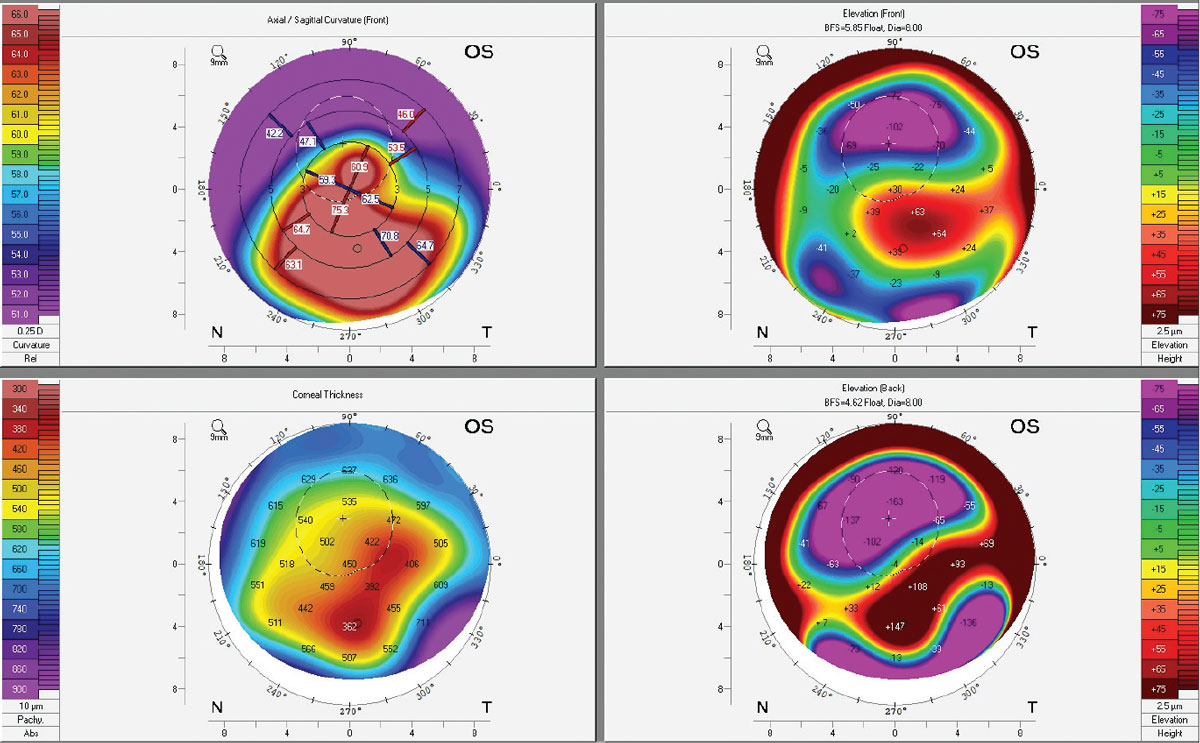 |
|
Corneal topography of an advanced keratoconus patient with an Intacs implant and corneal scarring. Note the extreme elevation differences of the back surface of the cornea, corresponding to the thinnest pachymetry areas. For additional diagnostic imaging on this patient, see photo series under subheading titled Patient Psychology. Click image to enlarge. |
Pathophysiology
While there are a few well-known factors concerning how keratoconus develops, they are less concrete than you may think, our experts shared.
“At the risk of oversimplifying: no rub, no cone.”—Eef van der Worp, PhD
A history of atopy, eczema, allergies, UV-light exposure and eye rubbing have long been implicated as environmental factors that play a role in the pathogenesis of keratoconus.37-39
While the overall findings have been inconclusive between studies of eye rubbing’s influence on keratoconus, it is widely accepted that there is a positive association between the two.40 The exact mechanism is still elusive. The rubbing must be prolonged and repetitive to sufficiently alter the biomechanics of the cornea—findings suggest the greater the force of rubbing, the more likely it is for progression and increase in severity—however, the timeframe and force required is not known at this point. The part of the body used to rub (knuckle or fingertip) can also potentially affect the amount of force applied.
Keratoconic patients tend to rub their eyes with their knuckles, thereby generating more force than non-keratoconic individuals who rub their eyes with their fingertips.41 There’s even perhaps a significant relationship between the dominant hand of the patient and the more severe keratoconic eye due to the hand itself being stronger.42
Further, there are other possible mechanical factors that can induce rubbing besides use of the hands, such as sleeping on your side and participating in sports such as wrestling that put patients at risk for mechanical friction.41 Keratoconic patients not only tend to rub more often than their non-keratoconic counterparts, they also tend to use more force when doing so.43 Patient age can also play a role in differences in frequency and force of rubbing; even with similar amounts of ocular allergies, adults were measured to rub less than teenagers.42
While there is still incomplete data proving direct causation of keratoconus from eye rubbing, the literature points to a correlation between the two.
“You just can’t predict hydrops.”—Renee Reeder, OD
 |
|
Habitual eye rubbing, particularly when performed with the knuckles, has long been associated with increased likelihood of keratoconus development. However, numerous other factors—including age, genetic profile, history of atopic dermatitis, UV light exposure and more—also play a part and should be teased out during a careful patient history. Photo: Getty Images. Click image to enlarge. |
Acute corneal hydrops is a relatively rare complication of keratoconus to which there are currently no anatomic predictive risk factors.44 However, there are a number of associations and theories. Occurring in around 3% of cases, hydrops has correlations with eye rubbing, young age at onset, vernal keratoconjunctivitis, atopy, male sex and Down syndrome.45-47 Additional studies have found associations with steeper keratometry, poorer Snellen visual acuity at time of diagnosis and earlier age of diagnosis as having strong associations with subsequent acute corneal hydrops development.48 There is even a study that evaluated ethnic associations and found that Pacific ancestry has a strong positive association while New Zealand European heritage has a negative association.49
In the literature right now, anterior segment OCT, in vivo confocal microscopy and ultrasound biomicroscopy are all being used to monitor and manage the condition. In a study done by Fuentes, et al., the investigators noted that the presence of hyperreflective anomalies at Bowman’s layer with marked stromal and epithelial thinning as measured on OCT were all findings that their keratoconic patients had prior to developing acute corneal hydrops.50 These are seen in patients who do not yet have stromal scarring, which is considered to be protective against development of acute corneal hydrops due to the increase in rigidity.50
While corneal hydrops can be difficult to predict in certain patients, the literature suggests that the presence of advanced keratoconus is at least associated with its development.
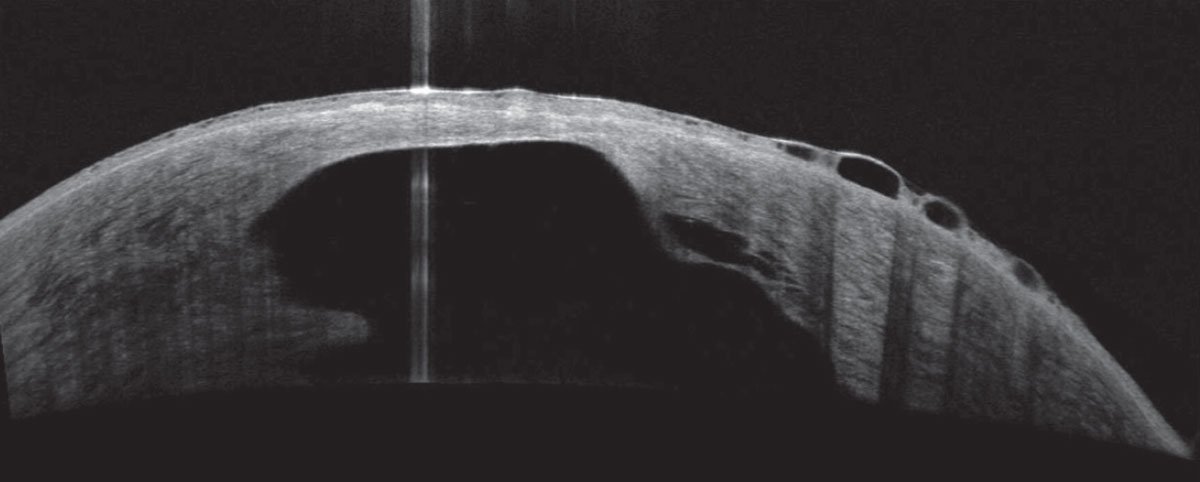 |
|
About 3% of patients will develop hydrops. The condition is correlated with eye rubbing, young age at onset, vernal keratoconjunctivitis, atopy, male sex and Down syndrome. Photo: Joseph Shovlin, OD. Click image to enlarge. |
Patient Psychology
Given its early onset in life, keratoconus is a formative experience for most patients. What does that do to their psychological make-up? Our experts share their observations.
“I am surprised by their personality.”—Abigael Chocron, OD
While the postulation that there’s a link between keratoconus and certain atypical personality traits has been suggested since the 1980s, there is at this time no concrete evidence of a unique “keratoconic personality” relative to high myopes.51 However, it’s possible that the perception of this unique personality is due to the specific interactions that patients have with their healthcare providers.
One study finds that these individuals tend to have maladaptive coping mechanisms, where they are less cooperative, less conforming and less respectful when interacting with their healthcare providers.52 Another suggested a “two-hit hypothesis” for developing the “perceived keratoconus personality.”51 The first concept is that the onset of visual deterioration occurs during a vulnerable period in the patient’s psychosocial development, when pathological coping mechanisms are poorly developed. The second is the disparate perceptions of what kind of burden keratoconus will have on the patient’s vision and livelihood.
These two experiences occurring in succession during this vulnerable and critical developmental timeframe for patients can potentially develop into a “perceived keratoconus personality.”
In another study, keratoconic patients were compared to a group of moderate to high myopes who also need contact lenses for visual correction. Results showed that the personality traits assessed—such as social introversion, paranoia and depression—were not significantly different between the two groups studied.53
“Patients will put up with anything to see.”—Priscilla Sotomayor, OD
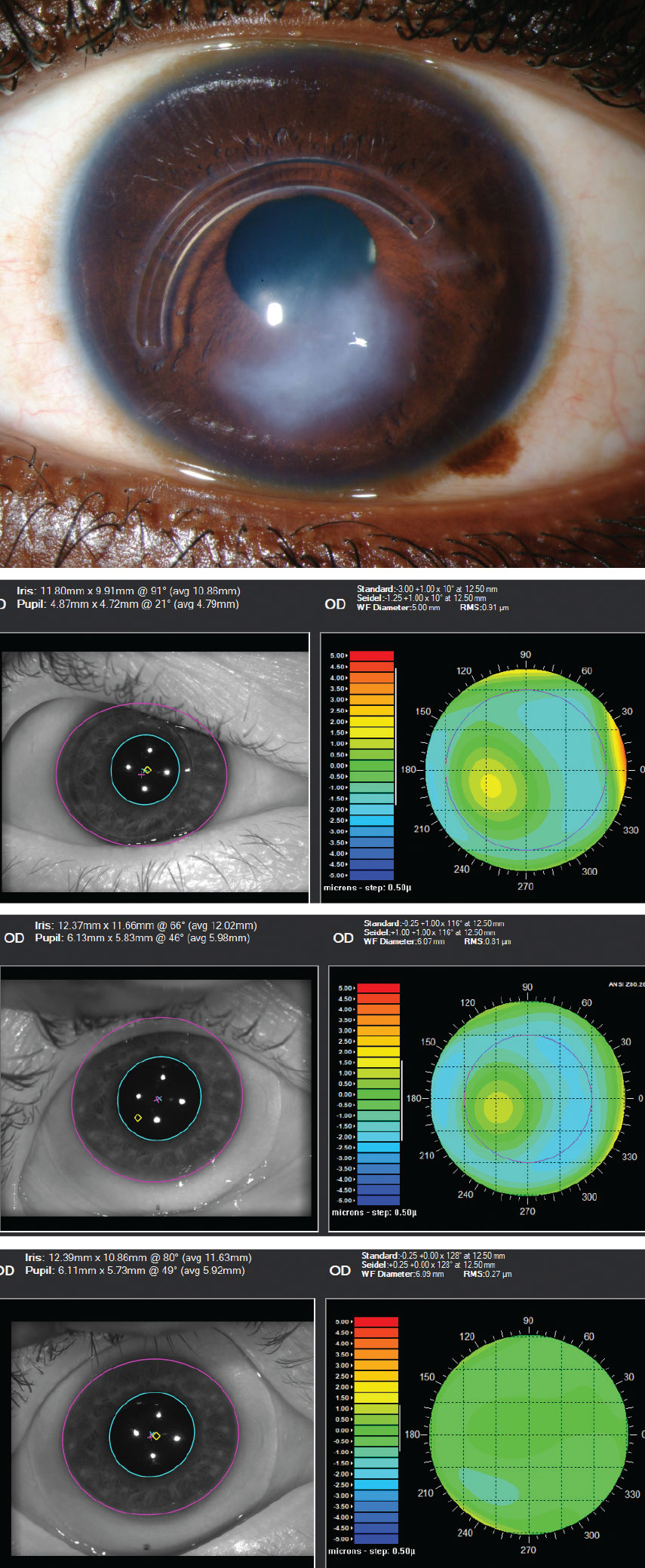 |
|
This series shows an advanced keratoconus patient with Intacs ring implantation and significant scarring. First image: slit lamp appearance. Second image: the impact of higher-order aberrations without correction (0.91µm@5mm pupil). Third image: aberrations remain with scleral lens wear (0.81µm@6mm pupil). Fourth image: aberration profile has been improved with use of an HOA-corrected scleral lens (0.27µ[email protected] pupil). Click image to enlarge. |
Since keratoconus is typically diagnosed during income-earning, peak education and child-rearing years, the condition has the potential to decrease vision-related quality of life.54 Keratoconic patients tend to experience significant ocular surface discomfort, perhaps exacerbated by contact lens wear, alterations in tear film, inflammation or corneal thinning with resultant nerve exposure.55,56 These patients can also experience ocular pain from stromal and epithelial damage and contact lens intolerance.56
Patients with keratoconus may develop various coping mechanisms for their condition, such as introversion, which can lead patients to become more withdrawn and passive as their keratoconus becomes more visually debilitating.52 In a study by Giedd et al., keratoconic patients were postulated to not be exacerbated by psychological stress when compared to the normative population of both physically ill and healthy adults due to the presence of low percentage of scores on measured psychogenic attitude scales.52
“There are people who meet these patients and think they’re crazy. There is sometimes a lack of empathy from practitioners to care for patients who have keratoconus.”—John Gelles, OD
The Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Observational Study showed that patient quality of life is low on all scales when using the National Eye Institute Visual Function Questionnaire (NEI-VFQ).57 In the follow-up study from the CLEK Observational Study group, quality of life scores continued to decline for patients whose visual acuity worsened and corneal curvature steepened.58
Although more recent advancements in scleral contact lenses and the introduction of CXL have emerged since the study concluded, there are still barriers to accessibility for patients who cannot afford or do not have access to eyecare providers who can offer those treatment options. Additionally, healthcare providers can potentially underestimate how the condition affects patients’ quality of life, especially if the keratoconus is clinically mild and the patient can achieve excellent Snellen visual acuity.51
In studies performed on clinical empathy, patients who perceive their doctors to be empathetic can potentially have better control of their treatment regimens, are less likely to sue for malpractice complaints and show objective changes in their immune system and significant reduction in duration and severity of symptoms.59-61 Empathetic concern itself is associated with a neural response in the brain’s striatum and ventromedial prefrontal cortex.62 When confronted with images of painful situations, physicians showed activation in their superior frontal gyrus, medial prefrontal cortex and temporoparietal junction, all of which contribute to activating executive attention and mental state understanding.62 Even though it can be perceived as blunting the instinct for empathy, expertise and learned experience play a role in how healthcare providers perceive others in pain by showing a downregulation in regions of the pain matrix when examining others’ suffering.63
“You need to have patient communication skills. Be that genius for them.”—Louise Sclafani, OD
In a study investigating how keratoconic patients receive prognostic and diagnostic information from their healthcare providers, results showed they were generally dissatisfied by the transmission of information from their doctors. This dissatisfaction can perhaps influence their perception of keratoconus and how it impacts their life.51
In order to improve the doctor-patient relationship, changing to a more patient-centered communication style by creating a sustainable relationship, verbalizing emotional experiences, exploring patients’ perspectives and developing strategies jointly can be beneficial.64-67 These skills can be further developed with training in conversation techniques. A couple examples of effective techniques include the NURSE model for handling emotions (naming, understanding, respecting, supporting, exploring) and the WEMS technique (waiting, echoing, mirroring, summarizing).68-70 It is possible for these patient-centered skills to be learned and improved by practitioners.71
Dr. Song received her OD degree at the SUNY College of Optometry and graduated with numerous awards, including the Chancellor’s Award for Student Excellence. She is currently completing a cornea and contact lens residency at the college. She has presented multiple posters at contact lens–focused conferences in eye care. Dr. Song has no financial disclosures. Dr. Sindt is a clinical professor of ophthalmology and visual sciences and director of the Contact Lens Service at the University of Iowa Carver College of Medicine. An internationally known expert in corneal disease and specialty lens design techniques, she is also Associate Clinical Editor of Review of Optometry and Review of Cornea & Contact Lenses. Dr. Sindt is the inventor and founder of EyePrintPro custom scleral contact lens manufacturing.
1. Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263-270. 2. Godefrooij DA, de Wit GA, Uiterwaal CS, et al. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169-172. 3. Olivares Jiménez JL, Guerrero Jurado JC, Bermudez Rodriguez FJ, Serrano Laborda D. Keratoconus: age of onset and natural history. Optom Vis Sci. 1997;74(3):147-151. 4. Millodot M, Ortenberg I, Lahav-Yacouel K, Behrman S. Effect of ageing on keratoconic corneas. J Optom. 2016;9(2):72-77. 5. Zhang H, Tian L, Guo L, et al. Comprehensive evaluation of corneas from normal, forme fruste keratoconus and clinical keratoconus patients using morphological and biomechanical properties. Int Ophthalmol. 2021;41(4):1247-1259. 6. Alió JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22(6):539-545. 7. Hashemi H, Beiranvand A, Yekta A, Maleki A, Yazdani N, Khabazkhoob M. Pentacam top indices for diagnosing subclinical and definite keratoconus. J Curr Ophthalmol. 2016;28(1):21-26. Published 2016 Mar 29. 8. Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akıncı A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013;39(9):1348-1357. 9. Maeda N, Fujikado T, Kuroda T, et al. Wavefront aberrations measured with Hartmann-Shack sensor in patients with keratoconus. Ophthalmology. 2002;109(11):1996-2003. 10. Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg. 2007;23(8):774-781. 11. Koh S, Inoue R, Maeno S, et al. Characteristics of higher-order aberrations in different stages of keratoconus. Eye Contact Lens. 2022;48(6):256-260. 12. Salman A, Kailani O, Marshall J, et al. Evaluation of anterior and posterior corneal higher order aberrations for the detection of keratoconus and suspect keratoconus. Tomography. 2022;8(6):2864-2873. 13. Greenstein SA, Chung D, Rosato L, Gelles JD, Hersh PS. Corneal higher-order aberrations after crosslinking and intrastromal corneal ring segments for keratoconus. J Cataract Refract Surg. 2020;46(7):979-985. 14. Mimouni M, Najjar R, Rabina G, Vainer I, Kaiserman I. Visual acuity in patients with keratoconus: a comparison with matched regular myopic astigmatism. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):313-319. 15. Lee HK, Jung EH, Cho BJ. Epidemiological association between systemic diseases and keratoconus in a Korean population: A 10-Year nationwide cohort study. Cornea. 2020;39(3):348-353. 16. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627. 17. Hayes S, O’Brart DP, Lamdin LS, et al. Effect of complete epithelial debridement before riboflavin-ultraviolet-A corneal collagen crosslinking therapy. J Cataract Refract Surg. 2008;34(4):657-661. 18. Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2017;17(1):262. 19. Miller C, Castro HM, Ali SF. Collagen crosslinking for keratoconus management in the pediatric population. Int Ophthalmol Clin. 2022;62(1):33-44. 20. Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-369. 21. Meyer JJ, Jordan CA, Patel DV, et al. Five-year results of a prospective, randomised, contralateral eye trial of corneal crosslinking for keratoconus. Clin Exp Ophthalmol. 2021;49(6):542-549. 22. Moschos MM, Gouliopoulos NS, Kalogeropoulos C, et al. Psychological aspects and depression in patients with symptomatic keratoconus. J Ophthalmol. 2018:7314308. 23. Morse AR. Vision function, functional vision, and depression. JAMA Ophthalmol. 2013;131(5):667-668. 24. Zhang X, Bullard KM, Cotch MF, et al. Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005-2008. JAMA Ophthalmol. 2013;131(5):573-581. 25. Moschos MM, Gouliopoulos NS, Kalogeropoulos C, et al. Psychological aspects and depression in patients with symptomatic keratoconus. J Ophthalmol. 2018;2018:7314308. Published 2018 May 29. 26. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. 27. Cubo E, Bernard B, Leurgans S, Raman R. Cognitive and motor function in patients with Parkinson’s disease with and without depression. Clin Neuropharmacol. 2000;23(6):331-334. 28. Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand. 2006;114(6):398-410. 29. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70. 30. Paz SH, Globe DR, Wu J, Azen SP, Varma R; Los Angeles Latino Eye Study. Relationship between self-reported depression and self-reported visual function in Latinos. Arch Ophthalmol. 2003;121(7):1021-1027. 31. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. 32. Schrag A, Jahanshahi M, Quinn NP. What contributes to depression in Parkinson’s disease? Psychol Med. 2001;31(1):65-73. 33. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267-273. 34. Giedd KK, Mannis MJ, Mitchell GL, Zadnik K. Personality in keratoconus in a sample of patients derived from the internet. Cornea. 2005;24(3):301-307. 35. Mazzotta C, Balestrazzi A, Traversi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26(4):390-397. 36. Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL. Keratoconus natural progression: a systematic review and meta-analysis of 11 529 Eyes. Ophthalmology. 2019;126(7):935-945. 37. Cristina Kenney M, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. 2003;26(3):139-146. 38. Gondhowiardjo TD, van Haeringen NJ, Völker-Dieben HJ, et al. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea. 1993;12(2):146-154. 39. Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. 40. Sahebjada S, Al-Mahrouqi HH, Moshegov S, et al. Eye rubbing in the aetiology of keratoconus: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2021;259(8):2057-2067. 41. Mazharian A, Panthier C, Courtin R, et al. Incorrect sleeping position and eye rubbing in patients with unilateral or highly asymmetric keratoconus: a case-control study. Graefes Arch Clin Exp Ophthalmol. 2020;258(11):2431-2439. 42. McMonnies CW, Boneham GC. Keratoconus, allergy, itch, eye-rubbing and hand-dominance. Clin Exp Optom. 2003;86(6):376-384. 43. Najmi H, Mobarki Y, Mania K, et al. The correlation between keratoconus and eye rubbing: a review. Int J Ophthalmol. 2019;12(11):1775-1781. 44. Maharana PK, Sharma N, Vajpayee RB. Acute corneal hydrops in keratoconus. Indian J Ophthalmol. 2013;61(8):461-464. 45. Tuft SJ, Gregory WM, Buckley RJ. Acute corneal hydrops in keratoconus. Ophthalmology. 1994;101(10):1738-1744. 46. Basu S, Vaddavalli PK, Ramappa M, Shah S, Murthy SI, Sangwan VS. Intracameral perfluoropropane gas in the treatment of acute corneal hydrops. Ophthalmology. 2011;118(5):934-939. 47. Grewal S, Laibson PR, Cohen EJ, Rapuano CJ. Acute hydrops in the corneal ectasias: associated factors and outcomes. Trans Am Ophthalmol Soc. 1999;97:187-203. 48. Fan Gaskin JC, Patel DV, McGhee CN. Acute corneal hydrops in keratoconus—new perspectives. Am J Ophthalmol. 2014;157(5):921-928. 49. Edwards M, Clover GM, Brookes N, Pendergrast D, Chaulk J, McGhee CN. Indications for corneal transplantation in New Zealand: 1991-1999. Cornea. 2002;21(2):152-155. 50. Fuentes E, Sandali O, El Sanharawi M, et al. Anatomic predictive factors of acute corneal hydrops in keratoconus: an optical coherence tomography study. Ophthalmology. 2015;122(8):1653-1659. 51. Mannis MJ, Ling JJ, Kyrillos R, Barnett M. Keratoconus and personality—a review. Cornea. 2018;37(3):400-404. 52. Giedd KK, Mannis MJ, Mitchell GL, Zadnik K. Personality in keratoconus in a sample of patients derived from the internet. Cornea. 2005;24(3):301-307. 53. Cooke CA, Cooper C, Dowds E, et al. Keratoconus, myopia, and personality. Cornea. 2003;22(3):239-242. 54. Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-232. 55. Dogru M, Karakaya H, Ozçetin H, et al. Tear function and ocular surface changes in keratoconus. Ophthalmology. 2003;110(6):1110-1118. 56.Gothwal VK, Gujar R, Sharma S, Begum N, Pesudovs K. Factors affecting quality of life in keratoconus. Ophthalmic Physiol Opt. 2022;42(5):986-997. 57. Kymes SM, Walline JJ, Zadnik K, Gordon MO; Collaborative Longitudinal Evaluation of Keratoconus study group. Quality of life in keratoconus. Am J Ophthalmol. 2004;138(4):527-535. 58. Kymes SM, Walline JJ, Zadnik K, Gordon MO; Collaborative Longitudinal Evaluation of Keratoconus study group. Quality of life in keratoconus. Am J Ophthalmol. 2004;138(4):527-535. 59. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. 60. Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent). 2003;16(2):157-161. 61. Rakel DP, Hoeft TJ, Barrett BP, Chewning BA, Craig BM, Niu M. Practitioner empathy and the duration of the common cold. Fam Med. 2009;41(7):494-501. 62. Decety J. Empathy in Medicine: What it Ii, and how much we really need it. Am J Med. 2020;133(5):561-566. 63. Coll MP, Grégoire M, Eugène F, Jackson PL. Neural correlates of prosocial behavior towards persons in pain in healthcare providers. Biol Psychol. 2017;128:1-10. 64. Zandbelt LC, Smets EM, Oort FJ, Godfried MH, de Haes HC. Medical specialists’ patient-centered communication and patient-reported outcomes. Med Care. 2007;45(4):330-339. 65. Puntis M. Skills for communicating with patients (2nd ed.). Ann R Coll Surg Engl. 2006;88(1):85-86. 66. Bensing J. Bridging the gap. The separate worlds of evidence-based medicine and patient-centered medicine. Patient Educ Couns. 2000;39(1):17-25. 67. Mead N, Bower P, Hann M. The impact of general practitioners’ patient-centredness on patients’ post-consultation satisfaction and enablement. Soc Sci Med. 2002;55(2):283-299. 68. Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med. 2007;167(5):453-460. 69. Langewitz W, Heydrich L, Nübling M, et al. Swiss Cancer League communication skills training programme for oncology nurses: an evaluation. J Adv Nurs. 2010;66(10):2266-2277. 70. Langewitz WA, Edlhaimb HP, Höfner C, et al. Evaluation eines zweijährigen curriculums in psychosozialer und psychosomatischer medizin—umgang mit emotionen und patientenzentrierter gesprächsführung. Psychother Psychosom Med Psychol. 2010;60(11):451-456. 71. Maatouk-Bürmann B, Ringel N, Spang J, et al. Improving patient-centered communication: Results of a randomized controlled trial. Patient Educ Couns. 2016;99(1):117-124. |