It’s Monday morning. As you’re reading over your patient schedule for the day, your receptionist asks if you can take a walk-in to start the morning: Mr. Jones woke up with a very painful right eye.
“Another recurrent corneal erosion!” you think to yourself. It’s the fourth one in the last 10 months.
You wish there were a better way to manage these erosions and help prevent future recurrence. You’ve heard about amniotic membranes; maybe that’s the solution you’re looking for.
But how exactly do you use one? In this article, the second of a six-part, print-and-video instructional series, we’ll show you how.
| |
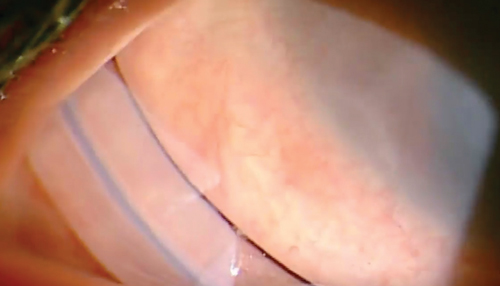
| Amniotic membrane grafts (such as the Prokera Slim shown here) promote epithelialization, decrease inflammation and scarring, prevent new blood vessel growth and improve comfort. |
What AMGs Can Do
Amniotic membrane grafts (AMG) were first introduced in eye care more than 60 years ago, but stable application to the eye was not successful for the management of many ophthalmic indications until 1995.1
The popularity of AMGs over the past two decades has grown immensely, perpetuated by their ability to speed healing and facilitate regeneration of ocular tissues.1-3
AMGs promote epithelialization, decrease inflammation and scarring, prevent new blood vessel growth and improve patient comfort by reducing pain.
The membrane is composed of three layers: a single layer of epithelial cells, a thick basement membrane, and an avascular stromal layer. The stromal layer is believed to help downregulate major inflammatory complexes that are found in many ocular surface conditions that can lead to scarring.4,5 While anti-inflammatory mediators can indirectly reduce scarring, amniotic membranes also have a direct anti-scarring effect through inhibition of fibroblasts at a transcriptional level.6
In addition to less scarring, corneal surfaces fit with an AMG have also shown reduced neovascularization. Amniotic membrane tissue is naturally avascular, and it inhibits the migration of vascular endothelial growth factor (VEGF). Prepared AMGs seem to have this same property also; they prevent the migration of VEGF, allowing the underlying cornea to receive the inherent antiangiogenic properties of the AMG.7
   | |
|
|
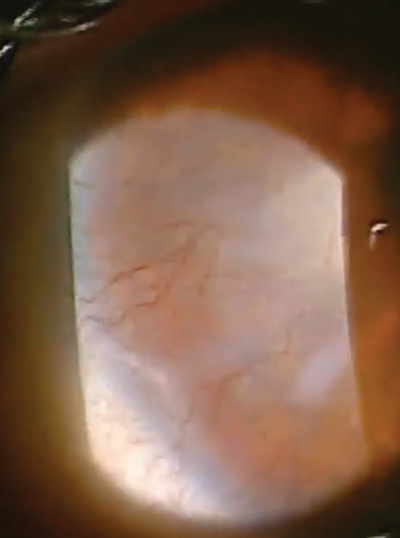
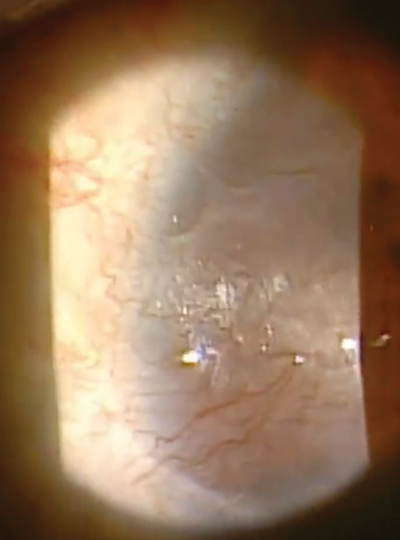
Our patient in this case is a 32-year-old female who had spilled bleach particles in both eyes due to a work-related accident. In the right eye, which was 20/400, she had a marginal ulcer as well as extensive neovascularization, haze and scarring. |
Types of AMGs
Since the discovery that AMGs accelerate corneal healing, three different types of AMGs have been developed: permanent surgical grafts, dehydrated sutureless grafts and cryopreserved sutureless grafts.
• Surgical grafts. Corneal surgeons use this type when a more permanent graft needs to be sutured onto the tissue, where it will later dissolve. This is commonly used during conjunctival reconstruction surgeries such as pterygium resection.
• Dehydrated sutureless grafts. These are becoming an increasingly popular treatment by optometrists and ophthalmologists for conditions that may lead to corneal scarring. Two of the most common are the AmbioDisk (IOP Ophthalmics) and BioDOptix (BioD).
Dehydrated sutureless grafts—a flat disc of tissue without a stabilizing outer ring—require the doctor to have slightly more finesse and dexterity during application. A lid speculum is required for applying the AMG to the cornea. After the graft is smoothed out and centered over the involved area, a bandage contact lens is applied over the top of the AMG. Special care must be taken when removing the lid speculum in order to not disrupt the graft by bumping it or the contact lens.
|
|
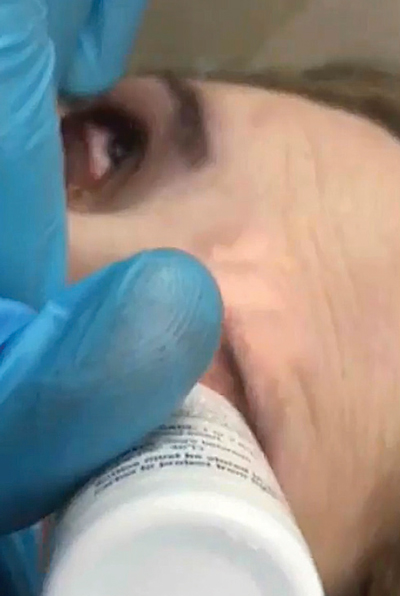
| The first step: instill anesthetic into the involved eye. |
|
• Cryopreserved sutureless grafts. Because dehydrated AMGs may be more challenging to insert and require a lid speculum, the third type of AMG, a cryopreserved sutureless graft—the Prokera (Bio-Tissue)—is a popular choice for optometrists interested in using this treatment in their practice.
The Prokera amniotic membrane is fastened within an ophthalmic conformer ring. All cell activity of the tissue has been inactivated to eliminate the possibility for graft rejection. It is stored in a medium that contains ciprofloxacin and amphotericin B, and must be kept cold during shipping and storage.8
Prokera AMGs come in three thicknesses: Prokera Slim (~100µm thick), Prokera and Prokera Plus (~200µm thick). The recommended thickness is based on the severity of the corneal defect being treated—the more severe the condition, the thicker the AMG must be. Prokera Slim is for mild to moderate indications (such as recurrent corneal erosion), Prokera is moderate to severe indications (neurotrophic epithelial defect) and Prokera Plus is for very severe indications (chemical burns). For most indications we use the Prokera Slim in our offices with good success and patient comfort.
Indications and Contraindications
Use of an AMG is clinically indicated for any condition causing damage to the ocular surface cells, underlying stromal inflammation or, most importantly, any condition that could lead to permanent scarring affecting the patient’s vision. Some of these include recurrent corneal erosion, corneal sequelae of severe dry eye syndrome, neurotrophic ulcer, persistent corneal epithelial defect, chemical and thermal burn, post-DSEK for bullous keratopathy, Salzmann’s nodular degeneration, acute Stevens Johnson syndrome, microbial ulcers, herpes simplex keratitis and herpes zoster keratitis.
|
|

|
|
|
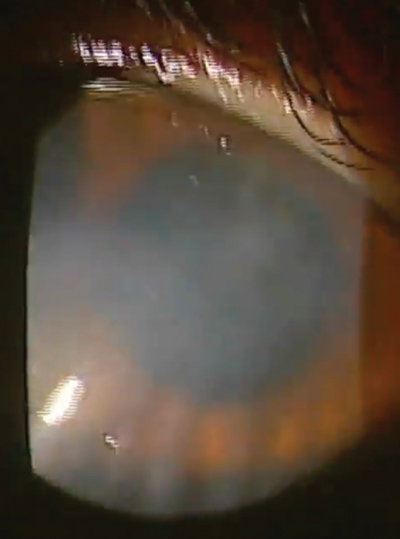
Because the graft is stored in the freezer, allow it to thaw for a few minutes before insertion. It’s OK to handle it gingerly with gloves. Forceps aren’t necessary. |
|
|
 |
|
|
Using sterile saline, thoroughly rinse off the storage solution from the graft. |
While a bandage contact lens may increase the incidence of microbial keratitis, no studies have demonstrated a similar occurrence with AMGs.9 Therefore, unlike a bandage contact lens, an AMG may be used at any point to treat the above conditions. Maintaining adjunct pharmaceutical therapy is important when active infection is present. So, instruct patients to continue their antibiotic drop right over the top of the AMG when indicated. (We’re not aware of any studies stating that topical absorption of medications is diminished by the presence of AMGs.)
There are two main contraindications for AMGs: patients with glaucoma drainage devices or filtering blebs. Other contraindications specific to Prokera include patient allergies to ciprofloxacin or amphotericin B.10
How to Insert the AMG
Because the Prokera has a conformer ring that makes handling relatively easy, application and removal is not a problem for most clinicians. Due to this, and because it’s readily available to most optometrists, this article focuses on the Prokera device.
When the AMG first arrives from the manufacturer, store it at an appropriate temperature based on when it may be used. It can be stored in a standard refrigerator for up to three months or stored in a freezer for up to one year.
Once a patient is a candidate for an AMG, remove the Prokera from the fridge or freezer, and let it sit at room temperature in its unopened package for at least 10 to 15 minutes.
To insert the device:
• First, instill one drop of anesthetic into the involved eye. Note: Some conditions, such as recurrent corneal erosions or Salzmann’s nodular degeneration, may benefit from corneal debridement before applying the Prokera.
• Once you open the package, be sure to handle the AMG with sterile gloves or forceps to maintain asepsis.
• Thoroughly rinse the AMG with sterile saline to remove the preservative storage solution. This will also increase initial patient comfort by reducing the stinging sensation.
• Hold the patient’s upper eyelid and instruct the patient to look down. Insert the Prokera into the superior fornix. Then, instruct the patient to look straight or slightly upward. While pulling down the lower eyelid, slide the Prokera into the inferior fornix.
• After the AMG is fully inserted, check for good centration of the AMG.
• Optional: Apply a tape tarsorrhaphy over the superior lid crease or lateral canthus to help keep the Prokera centered and minimize discomfort; however, many practitioners find this unnecessary when using the Prokera Slim. The necessity and duration of the tape tarsorrhaphy varies among individual patients and their pain threshold.
Follow-up depends on the ocular condition. For example, if treating a central bacterial corneal ulcer, patient follow-up would likely be every one to two days while using an appropriate antibiotic medication.
|
|
 |
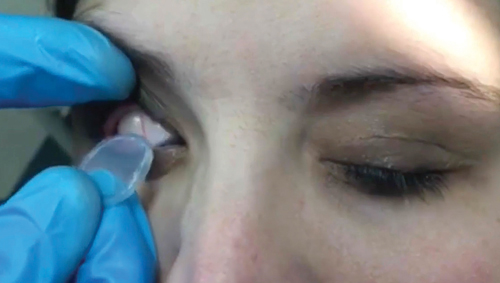
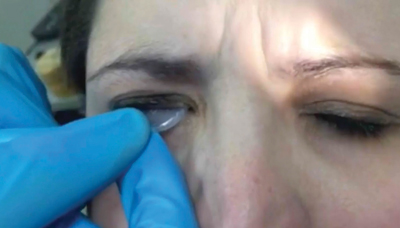
| To insert the graft, hold the upper eyelid and ask the patient to look down. | Slide the device into the superior fornix. The patient may feel some discomfort. | Ask the patient to look upward. Pull down the lower eyelid and slide the graft into the inferior fornix. |
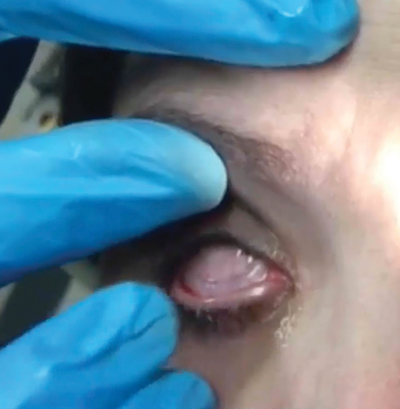 |
| |
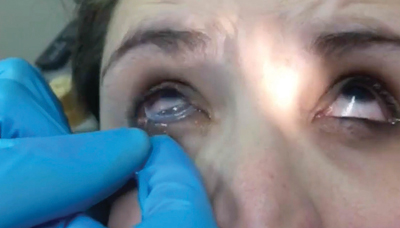
| Ask the patient to blink gently and then check for centration. | A tape tarsorrhaphy can lessen the discomfort of blinking. |
How to Remove the AMG
FDA regulations require that a sutureless amniotic membrane graft cannot stay on the eye for more than 29 days. That’s not usually a concern with an AMG. In most cases, it will dissolve in five to 10 days depending on the severity of the condition. For instance, in a severe chemical burn with intense inflammation, the AMG will likely dissolve quickly in a matter of three to four days. On the other hand, a neurotrophic ulcer that has little inflammation may need the AMG for two to four weeks to fully heal. For most conditions, the Prokera is on the ocular surface five to seven days.
Once the AMG dissolves, remove the conformer ring. If the AMG is still intact, but the ocular condition has fully healed, then remove the conformer ring and remaining AMG.
To remove the Prokera:
• First, instill one drop of topical anesthetic.
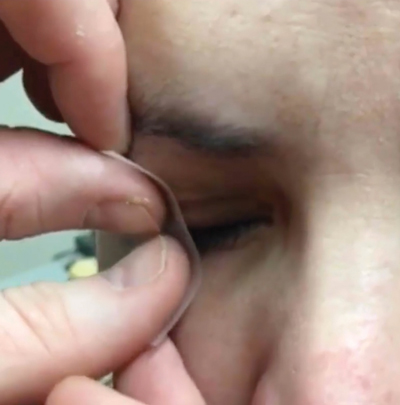
• Pull the lower eyelid down and instruct the patient to look up. Lift the lower edge of the Prokera with blunt forceps.
|
|
| The graft’s conformer ring is visible in the lateral canthus. Once the amniotic membrane graft dissolves (usually within about a week) and the eye has healed, remove the ring. |
|
• Instruct the patient to look down and apply a gentle pressure in a downward motion on the superior eyelid. This allows you to pull the Prokera down and off the globe.
• While no other lubrication drops or saline are necessary, preservative-free artificial tears may be instilled after removal to improve patient comfort.
For patients battling recurrent corneal erosions or severe dry eye syndrome, do not let them continue to suffer using standard treatments. What about those patients with central corneal ulcers or chemical burns and the threat of permanent vision loss? Consider an AMG.
By improving a patient’s severe corneal condition and restoring their quality of life, you’ll gain the patient’s deepest loyalty. If you’re able to provide a treatment that reduces scarring, promotes healing, decreases pain and helps to preserve vision, these patients will open the door to a number of new referrals.
Dr. Kyle Ryff is a graduate of the Southern California College of Optometry and is currently completing an Ocular Disease and Family Practice Residency at the Oklahoma College of Optometry with an emphasis in disease management and anterior segment laser procedures.
Dr. Brianna Ryff graduated from the Southern California College of Optometry and is the Cornea and Contact Lens resident at the Oklahoma College of Optometry. She is joining a private practice in Tempe, AZ to fit specialty contact lenses.
|
Coding Connection: Coding for Prokera
By John Rumpakis, OD, MBA, Clinical Coding Editor Inserting a Prokera may seem as simple as applying a bandage contact lens—but take care when coding and billing, or you may make an alarming mistake. Amniotic products are used in medicine for a wide array of problems, and the eye is no different. The American Medical Association created CPT Code 65778 (currently defined as: “Placement of amniotic membrane on the ocular surface; without sutures,”with a 10-day global period) because it recognized the importance of delivering the wound healing properties of cryopreserved amniotic membrane to the ocular surface without the use of sutures. Subsequently, the Center for Medicare and Medicaid Services (CMS) authorized payment policies for the procedure to be performed in both facility and non-facility settings, and all local Medicare carriers established coverage policies for this procedure. Although CPT references it as a surgical procedure, clinical application of a Prokera amniotic membrane device is virtually identical to the insertion of a bandage contact lens. So can this “surgical procedure” be performed by an optometrist? The answer is, it depends. Most state boards of optometry have deemed this procedure to be well within the optometric scope of practice. In fact, a recent consideration by CMS on the use of amniotic membranes for ocular surface disease states: “our medical advisors indicated that the procedure described by CPT code 65778 is not significantly different than placing a bandage contact lens on the surface of the eye to cover a corneal epithelial defect. CPT code 65778 describes the simple placement of a special type of bandage (a self-retaining amniotic membrane device) on the surface of the eye, which would most commonly be used in the HOPD [hospital outpatient department] to cover the surface of the eye after a procedure that results in a corneal epithelial defect.”1 Keep in mind that for CMS, a separate charge and reimbursement for the supply of the amniotic membrane is not allowed; it’s bundled into the reimbursement for the procedure itself (not unlike the rationale used for punctal plugs). However, other commercial carriers may have policies that allow for reimbursement of the procedure and the materials, and if so, the appropriate HCPCS Level II code is V2790 (“Amniotic membrane for surgical reconstruction, per procedure.”) Most importantly, because this is considered to be a minor surgical procedure, and in accordance with minor surgical rules, an office visit (either 920XX or 992XX) is generally not separately billable when performed on the same date of service as CPT code 65778. That’s because reimbursement for the 65778 code itself already includes compensation for the office visit related to the decision to perform this minor surgical procedure. So it would be the rare occasion to append modifier -25 to an E/M office visit performed on the same day as the application of a Prokera. Question or comments? E-mail [email protected]. 1. Ocular Services: Placement of Amniotic Membrane (APC 0233). Federal Register. Rules and Regulations. 2012 Nov 15;77(221): 68339-41. Available at: http://69.175.53.6/register/2012/Nov/15/2012-26902.pdf. |
Dr. Lighthizer is the assistant dean for clinical care services, director of continuing education, and chief of both the specialty care clinic and the electrodiagnostics clinic at the Oklahoma College of Optometry.
1. Liu J, Sheha H, Fu Y, et al. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010 October; 5(5): 645-61. The authors have no conflict of interest or financial relationship with Bio-Tissue.2. Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Scarless wound healing in mammalian fetus. Surgery. 1992; 174:441-51.
3. Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg. 1994; 220:10-18.
4. Heiligenhaus A, Meller D, Meller D, Steuhl K-P, Tseng SCG. Improvement of HSV-1 necrotizing keratitis with amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 2001;42:1969-74.
5. He H, Li W, Chen SY, et al. Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci. 2008; 49(10):4468-75.
6. Tseng SCG, Li D-Q, Ma X. Suppression of transforming growth factor isoforms, TGF-β receptor II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179:325-35.
7. Jiang A, Li C, Gao Y, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006; 25(10 Suppl. 1):S36-S40.
8. Prokera Slim Product Insert. Bio-Tissue website: www.biotissue.com/downloads/prokera-slim-insert_PI-BT-004E_V1.pdf. Accessed Dec 10, 2014.
9. Kent HD, Cohen EJ, Laibson PR, et al. Microbial keratitis and corneal ulceration associated with therapeutic soft contact lenses. CLAO J. 1990;(16):49-52.
10. Prasher P, Lehmann JD, Aggarwal NK. Ahmed tube exposure secondary to prokera implantation. Eye Contact Lens; 34 (4); 244-5

