 |
How to Conduct a Good Dry Eye Assessment
Perform a comprehensive evaluation and you'll be well on your way to the proper diagnosis.
By Jennifer Gould, OD
Release Date: March 15, 2021
Expiration Date: March 15, 2024
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Assess patients for dry eye disease.
- Review the clinical usefulness and real-world applications of DED questionnaires.
- Describe what the various dry eye diagnostic tools reveal about dry eye status.
- Distinguish between DED and conditions that present with similar symptoms.
- Educate patients on their disease.
Target Audience: This activity is intended for optometrists engaged in routine eye care and ocular surface disease management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Jennifer Gould, OD, SUNY College of Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 71557-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Author: Dr. Gould receives consulting fees from Aerie Pharmaceuticals and Carl Zeiss Meditec.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
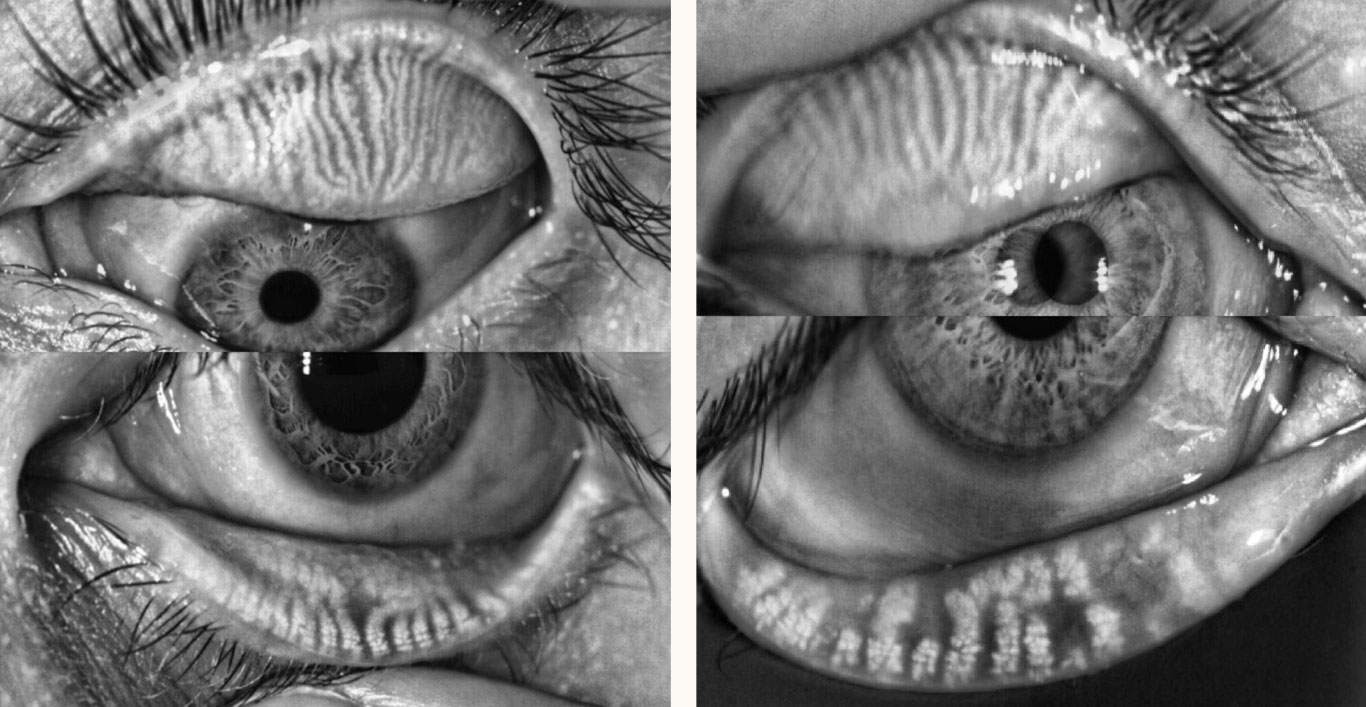 |
| Fig. 1. These LipiView II meibography images show a normal meibomian gland anatomy of upper and lower eyelids (left) and normal upper lid meibomian gland anatomy with meibomian gland dilation and atrophy of the lower eyelid (right). Click image to enlarge. |
While dry eye disease (DED) is common, proper diagnosis can be a challenge. Signs and symptoms don’t always correlate, and patients can present with a wide range of symptoms, if they have any at all. Clinicians should be prepared to assess patients carefully for DED, which involves a combination of subjective and objective measures.
Proper diagnosis of DED requires an extensive understanding of the condition, detailed case history including symptom and risk factor assessment, and a systematic clinical evaluation. This detailed-oriented examination will aid in refining the subtype of DED and, therefore, in determining appropriate management of the condition.
During a dry eye evaluation, it is also important to rule out other ocular conditions that have similar signs and symptoms to ensure successful management. This article will provide a comprehensive discussion about the etiology of DED as well as aspects of evaluating and caring for symptomatic and asymptomatic patients.
Understanding DED
Classically, dry eye is characterized by inflammation of the eyelids, ocular surface and/or lacrimal gland.1 DED is a source of ocular pain, irritation and tear film instability leading to visual fluctuations. These symptoms inevitably result in decreased quality of life for those coping with the disease.1,2
In 2017, the Tear Film and Ocular Surface Society (TFOS) published the Dry Eye Workshop (DEWS) II report with an updated definition of DED, which provides clues to the clinical picture of the condition. These clues—e.g., symptoms, osmolarity, inflammation—are pivotal in guiding patient evaluation and determining the subtype of DED.3
We know from the TFOS DEWS II definition that DED results when the homeostasis of the tear film is not maintained. In a balanced environment, the tear film provides a clear, smooth refractive surface that protects the cornea.4 Osmolarity is an indicator of the level of homeostasis of the tear film. Many factors can disrupt ocular surface homeostasis, including tear film instability, hyperosmolarity and ocular surface inflammation. A hyperosmolar state (i.e., loss of homeostasis) triggers an inflammatory cascade resulting in corneal epithelial and goblet cell loss (the latter effect impairing mucin formation). Clinically, the loss of homeostasis can be seen through variable patient symptoms of DED, punctate epitheliopathy, conjunctival staining and tear film instability.
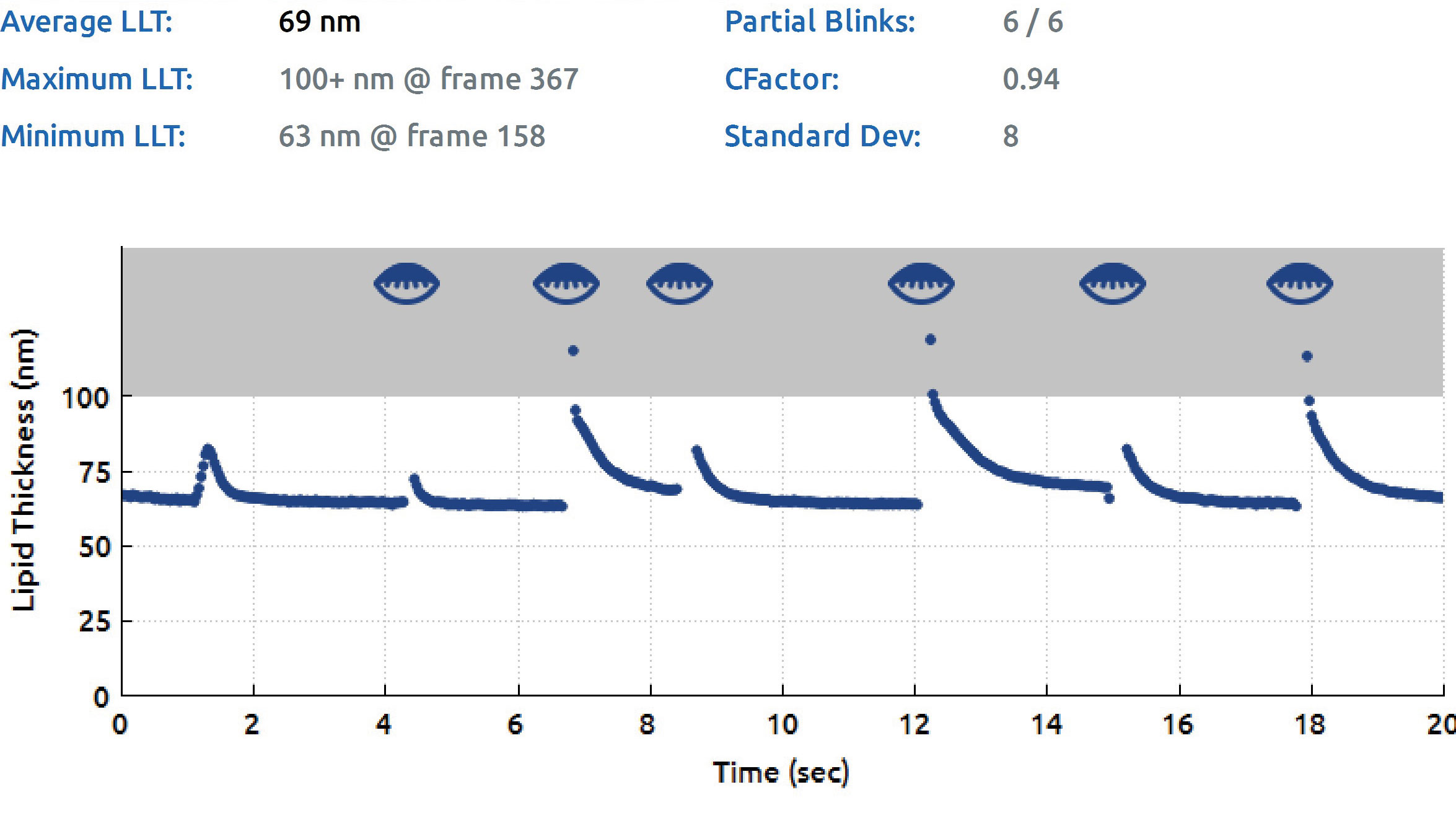 |
| Fig. 2. LipiView II Surface Interferometer lipid layer thickness (LLT) and blink rate assessment. Results show reduced average LLT and high partial blink rate contributing to meibomian gland dysfunction. Click image to enlarge. |
DEWS II recognized the neurosensory component of DED, a novel concept within dry eye and one not fully understood at this point in time. There are two types of ocular pain associated with DED: nociceptive and neuropathic. Nociceptive pain is an activation of sensory nerves secondary to ocular tissue damage that results in ocular pain. Neuropathic pain results from neurological defects and has been described as “pain without stain.”5 These patients report symptoms consistent with dry eye but do not have correlating clinical signs. Patients with DED can also have some neuropathic symptoms, including spontaneous pain, sensitivity to light and wind, and an exaggerated sensation of pain response (hyperalgesia).5,6 These neuropathic symptoms will remain even after a topical anesthetic is instilled during a clinical exam and can help to differentiate between nociceptive and neuropathic pain.6
It is also important to remember that chronic dry eye also causes corneal hypoesthesia.7 This can explain the mismatch often noticed in patients with DED: minimal symptoms with severe signs of dryness on our clinical examination.7 Corneal hypoesthesia can also be secondary to other conditions such as diabetes, previous herpetic keratitis, corneal surgery (such as LASIK) and long-term contact lens use.5,8 These other conditions should be considered when evaluating our patients with DED.
Dry eye disease can be broken into two major etiological categories: evaporative (EDE) and aqueous-deficient (ADDE). Some patients also have mixed-mechanism dry eye, which combines both etiological categories of the disease.3 The most common type of evaporative dry eye occurs secondary to meibomian gland dysfunction (MGD), a subtype of posterior blepharitis. It has been reported that 86% of patients with DED have MGD; this includes patients with either standalone EDE or mixed-mechanism DED.9
The most common cause of ADDE is inflammatory infiltration of the lacrimal gland. This ADDE can be associated with autoimmune disorders, such Sjögren’s syndrome or non-Sjögren’s autoimmune conditions. This inflammation of the lacrimal gland can result in a neurosensory block, further reducing aqueous release.
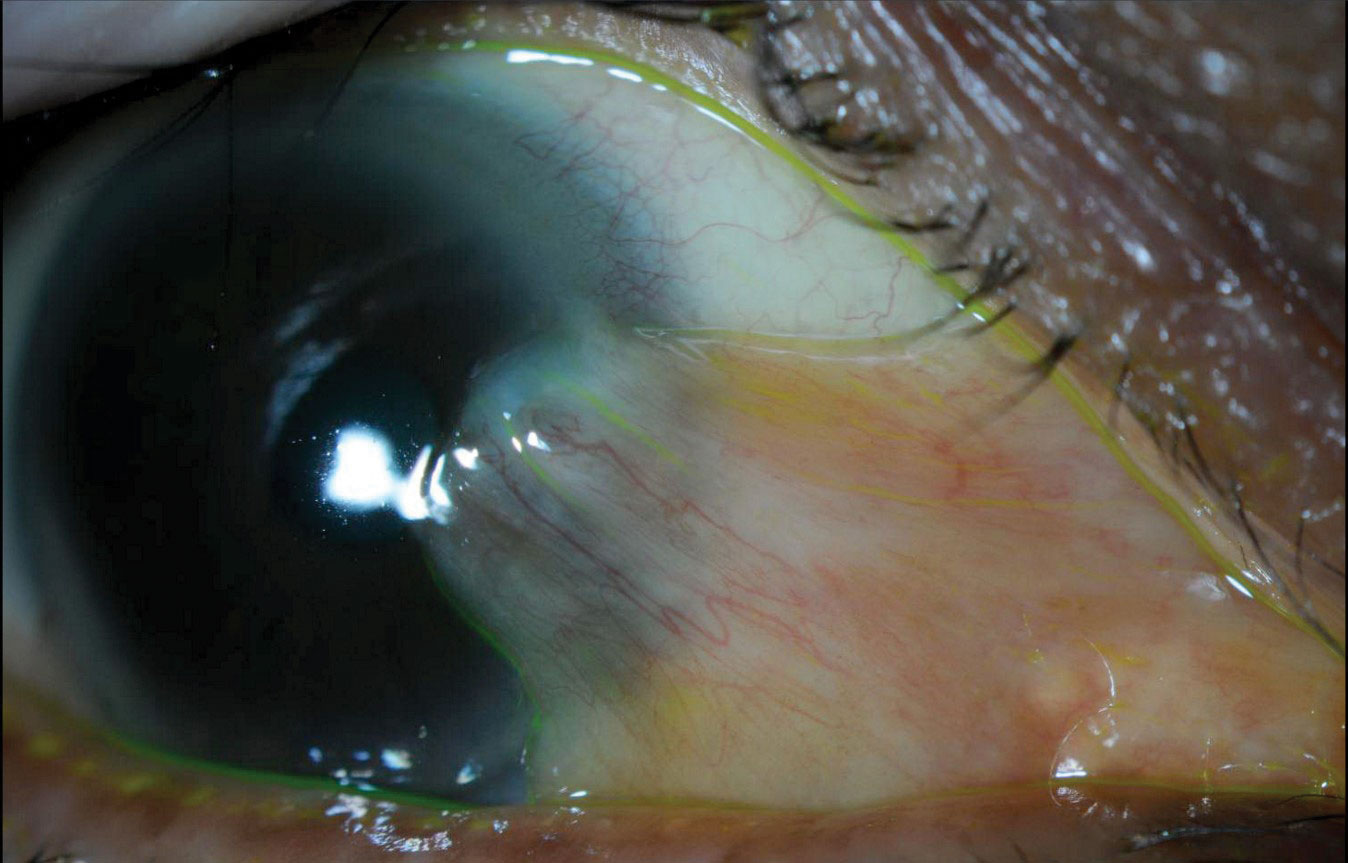 |
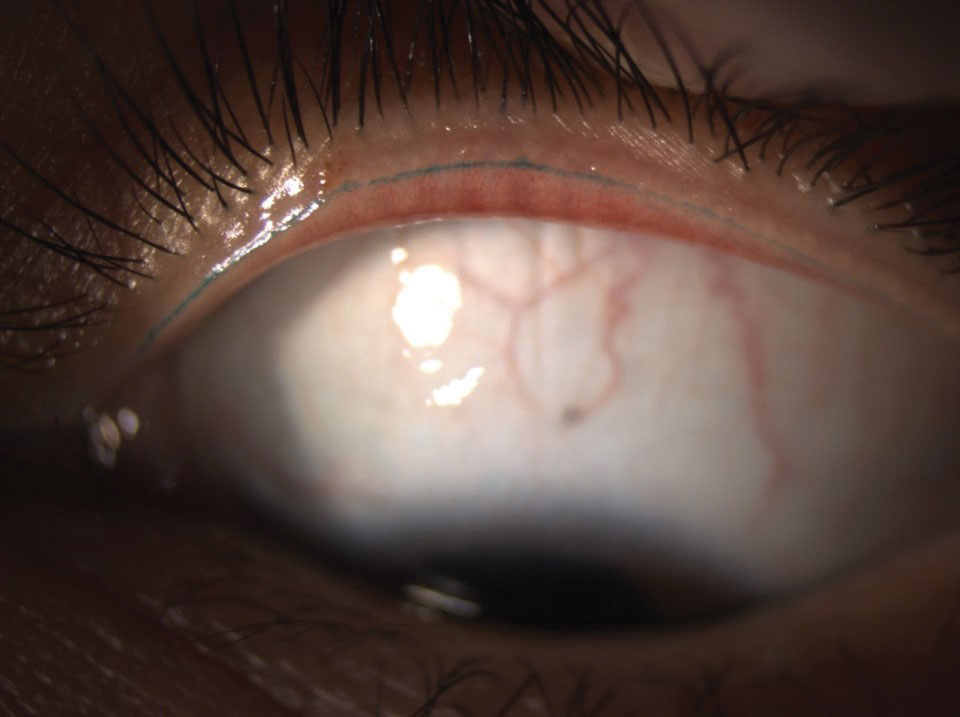
| Fig. 3. Slit lamp photo demonstrating large, nasal, mildly inflamed pterygium and inferior lid scalloping secondary to MGD. In addition to managing the MGD-associated DED, we referred this patient for pterygium removal due to persistent ocular irritation. Click image to enlarge. |
Assessment and Evaluation
It is important to take a systematic approach to dry eye patients with both objective and subjective testing during an evaluation. The DEWS II report and the American Society of Cataract and Refractive Surgery (ASCRS) algorithm for evaluating DED provide detailed test interpretation and methodical guides to aid eye care providers in identifying the disease.10,11
The ASCRS algorithm acknowledges the importance of effectively diagnosing and managing DED prior to cataract and refractive surgeries. It reports that properly treating ocular surface disorders improves visual outcomes and patient satisfaction postoperatively. This same concept can be applied to an optometric practice and can improve patient outcomes with enhanced refractions and reduced contact lens intolerance.
The ASCRS algorithm recommends a two-part DED evaluation: (1) screening of all patients for DED with symptom surveys and point-of-care testing and (2) clinical examination. Before this algorithm can be applied, a comprehensive ocular and medical history must be obtained.
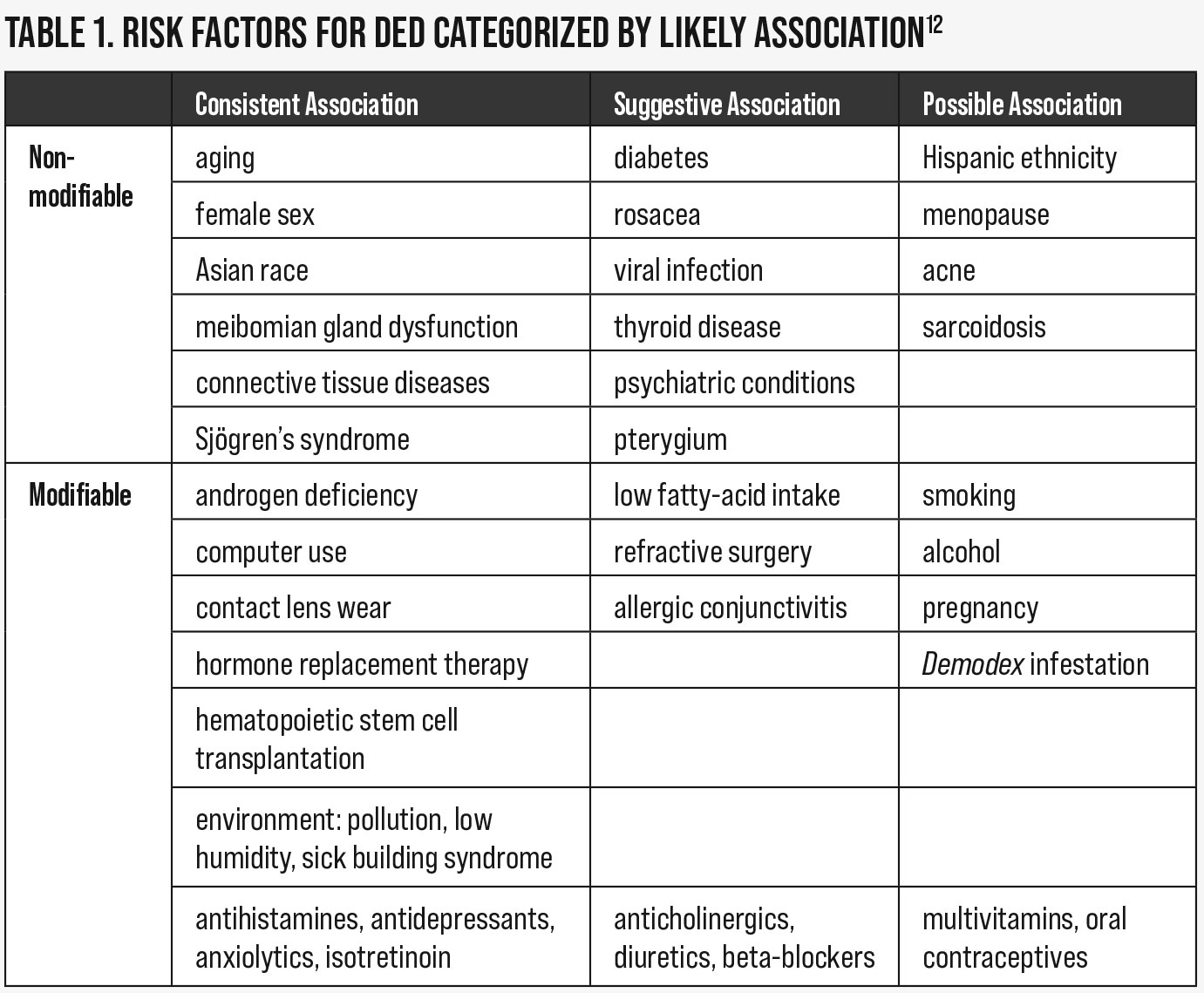
Directed case history to identify DED. Case history is important during a dry eye evaluation to properly assess risk factors and rule out iatrogenic causes of dry eye. Risk factors can be modifiable or non-modifiable and can help to elicit the etiological subtype of a patient’s condition (Table 1).12 For example, patients with a history of LASIK have some level of corneal anesthesia, leading to ADDE. All risk factors should be discussed with patients to aid in comprehension of the multifactorial nature of this chronic condition.
Some of these risk factors can be easily added as supplemental questions to a symptom survey, while others can be incorporated into your case history (e.g., medication reconciliation, computer use) or clinical exam (e.g., MGD, Demodex folliculorum evaluation).
Iatrogenic conditions result from some form of medical or cosmetic treatment. Medication-related iatrogenic dry eye can result from both systemic and ocular drug use. Table 2 shows a comprehensive list of systemic medications that have been shown or reported to be associated with DED in the DEWS II iatrogenic report.13 Many of these medications are very commonly prescribed and taken by our patients, and include analgesics such as aspirin or antihypertensive medications such as hydrochlorothiazide. Different classifications of drugs have different effects on the tear film, a few of which are worth noting.
Anti-cholinergic medications (e.g., antihistamines, antidepressants) affect muscarinic receptors in the lacrimal gland and conjunctival goblet cells and reduce overall aqueous and mucin production. Some drugs, including aspirin and chloroquine, are secreted into the tear film leading to ocular irritation and increased evaporation of the tear film. Retinoids like isotretinoin (used to treat severe acne) affect the epithelium of the meibomian glands and result in increased lipid secretion, tear osmolarity and tear film instability.
Topical ophthalmic drugs can have allergic, toxic and inflammatory effects on the ocular surface. This can result in tear film disruption, reduced aqueous secretion and/or neurotoxicity of the corneal nerves. Benzalkonium chloride (BAK), used to preserve many topical ophthalmic medications, has been shown to have a direct relationship on tear film osmolarity.13 Glaucoma patients have a high prevalence of dry eye associated with their glaucoma therapy; the more medications a patient is taking for glaucoma, the higher the prevalence of DED.13 This is primarily due to the preservatives (often BAK) within the drops.
Some of the other causes of iatrogenic DED include contact lens wear, ocular/lid surgeries, eyelid tattooing and noninvasive ventilation. Contact lens–associated dry eye is a result of a reduced and disrupted lipid layer, lower basal tear turnover, increased tear osmolarity and decreased tear meniscus volume.13 Common ocular surgeries that can contribute to DED include LASIK, PRK, cataract and blepharoplasty.13
Refractive surgeries (e.g., LASIK, PRK) lead to sensory deficit of the corneal nerves, resulting in decreased lacrimal aqueous distribution.13,14 Eyelid tattooing leads to meibomian gland atrophy, chronic conjunctival inflammation, decreased tear breakup time (TBUT) and increased fluorescein staining.13 The use of continuous positive airway pressure machines for the treatment of obstructive sleep apnea has been shown to increase ocular irritation, based on the Ocular Surface Disease Index (OSDI), and decrease TBUT.15
 |
| Click table to enlarge. |
Symptom surveys and point-of-care testing. We understand that signs and symptoms typical in dry eye care have variable correlation. For this reason, it is important to screen all patients for DED, as not all patients will be symptomatic. The ASCRS algorithm recommends using a symptom survey and point-of-care testing for this initial screening. The results of this screening can help guide other aspects of the clinical exam based on the findings.
As symptoms are very subjective and difficult to standardize and quantify, it is important to use a symptom survey when evaluating patients for DED.10,16 The questionnaire can be used both in the initial workup of a patient and also repeated to evaluate the efficacy of treatment. Many questionnaires have been created. The DEWS II report recommends using a questionnaire that has been validated, such as the OSDI, Standard Patient Evaluation of Eye Dryness (SPEED), Dry Eye Questionnaire (DEQ) or Contact Lens Dry Eye Questionnaire (CLDEQ).
The OSDI survey combines questions related to symptoms, quality of vision and environmental triggers. This questionnaire has been the most widely accepted for research purposes, and many of the other surveys are validated against it. For this reason, the DEWS II report recommends the OSDI.10 The ASCRS protocol uses a unique version of the SPEED survey that has an additional, non-validated question intended to ascertain the patient’s personality type to aid in understanding their expectations and approaching patient education.11
We know from the definition of DED that both inflammation and hyperosmolarity play key roles in the condition. Point-of-care testing can be used to evaluate patients for the presence of these DED factors, especially in asymptomatic patients.11 Two point-of-care tests are clinically available: InflammaDry (Quidel) and TearLab Osmolarity System (TearLab). Both have reimbursable CPT codes associated with them; they are 83516 and 83861, respectively.
InflammaDry provides an indication of whether or not inflammation is present within the tear film. Matrix metalloproteinase-9 (MMP-9) is an enzyme that is released into the tear film secondary to ocular inflammation. InflammaDry is a qualitative test that detects MMP-9 levels within the tear film. A positive result (presence of MMP-9) indicates that anti-inflammatory therapy may be useful in relieving patient symptoms and improving signs of DED. This should also be considered prior to the use of punctal plugs, as they will retain inflammatory mediators on the ocular surface, exacerbating signs and symptoms.
The TearLab Osmolarity System provides a qualitative measurement of the osmolarity of the tear film. The company reports that a measurement greater than 308mOsm/L or an inter-eye variability of greater than 8mOsm/L is considered abnormal. Since osmolarity can be increased in both aqueous-deficient and evaporative dry eye, the test result does not narrow down the etiology. However, it can help to differentiate it from other conditions that result in similar symptoms, such as allergic conjunctivitis, anterior blepharitis, conjunctivochalasis, computer vision syndrome and epithelial basement membrane dystrophy (EBMD).17 In these conditions, symptoms will be variably present, but osmolarity will be normal (less than 308mOsm/L).
According to the ASCRS algorithm, if one of the three components (questionnaire, InflammaDry, TearLab osmolarity measurement) is abnormal, DED is likely and further testing should be done to identify the etiology to aid in patient management. In addition to the screening, other noninvasive tests such as meibomian gland imaging, lipid layer thickness (LLT) evaluation and noninvasive tear breakup time (NI-TBUT) can also be performed to aid diagnosis.
Meibomian gland imaging can be performed using many different types of equipment, including the LipiScan Dynamic Meibomian Imager (Johnson & Johnson Vision Care), the LipiView II Ocular Surface Interferometer (Johnson & Johnson Vision Care) and the Keratograph 5M (Oculus). These devices allow for high-definition images of the meibomian glands to assess gland dropout, dilation and tortuosity, which, if present, aids diagnosis of MGD (Figure 1).
In addition to meibomian gland imaging, other measurements such as LLT, blink quality assessments and NI-TBUT can also be gathered using the LipiView II (Figure 2) and the Oculus Keratograph 5M. LLT allows the provider to understand the amount of meibum being excreted. A scant lipid layer can be due to MGD or abnormal blinking. For patients with reduced or low LLT (i.e., less than 100nm), lipid-based tears may be recommended.18 If the lipid layer alteration is associated with lagophthalmos (partial blinking), blinking exercises can also be used to supplement DED therapy.19
 |
| Click table to enlarge. |
Clinical Evaluation
Once it has been determined that a patient likely has dry eye based on a screening, further clinical examination should be performed. The ASCRS algorithm lays out a simple, organized approach for ocular surface evaluation to confirm the subtype, severity and visual significance: look, lift, pull and push.11 This four-step process is performed in addition to using vital dyes to better visualize the anterior segment and can be supplemented with other quantitative tests of tear film quality.
Look. During a slit lamp examination, many structures and conditions should be evaluated to rule in or out conditions associated with DED. The following is a non-exhaustive list of items that should be evaluated:
- Blink quality and tear meniscus height.
- Lid, lash and/or globe abnormalities (e.g., entropion, ectropion, trichiasis, proptosis).
- Anterior blepharitis (e.g., scurf, collarettes related to a Demodex folliculorum infestation, frothy tears).
- Posterior blepharitis (e.g., lid margin keratinization, capped meibomian glands, telangiectasias, scalloping of the lid margin as seen in Figure 3).
- Conjunctival abnormalities (e.g., injection, follicles, papillae, conjunctivochalasis, pinguecula).
- Corneal abnormalities (e.g., pterygia, EBMD, filamentary keratopathy, superficial punctate erosions, superior limbic keratopathy).
Lift and pull. The superior cornea should be evaluated while lifting the upper lids up. EBMD and superior limbic keratopathy can be easily missed by omitting this step during a slit lamp examination. In addition, the upper and lower eyelids should be slightly displaced from the globe to observe for potential rebound. This will aid in noting lid laxity associated with floppy eyelid syndrome.
Push. Light pressure should be applied to the lower lid margin to assess the meibum secreted from the meibomian glands. This can be done using your finger, a cotton tip applicator or the Meibomian Gland Evaluator (Johnson & Johnson Vision Care). This device mimics the pressure applied on the meibomian glands during a natural blink. Abnormal meibum secretion upon palpation is highly suggestive of MGD and, therefore, is an important part of each work-up.20
 |
| Fig. 4. Slit lamp image showing diffuse punctate epithelial fluorescein staining secondary to lagophthalmos. Superior to the staining, the tear film has also “broken” as visualized during TBUT testing with fluorescein dye. Photo: Alexander Kabirir, OD. Click image to enlarge. |
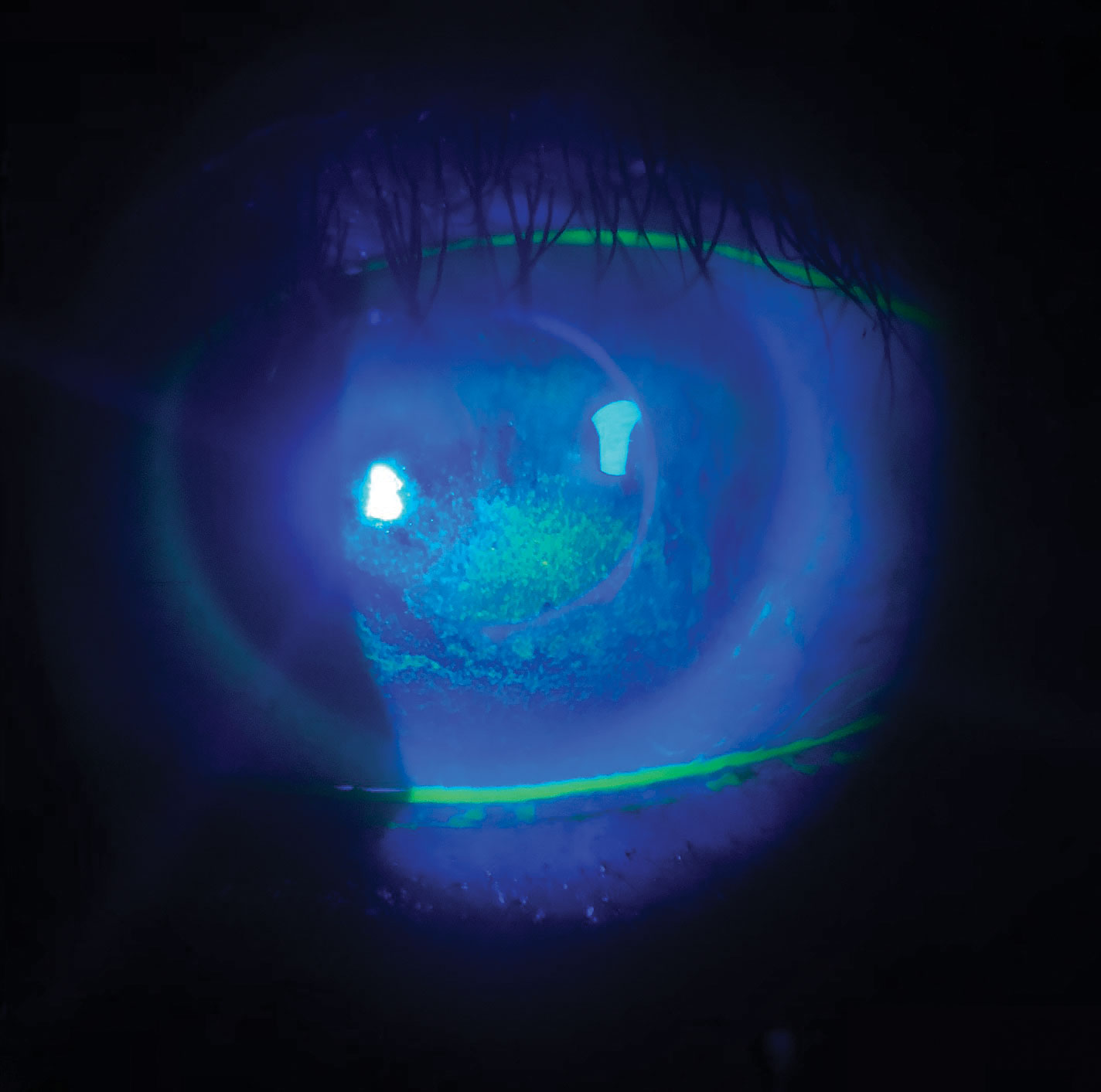
Vital dye staining. Fluorescein dye should be instilled as part of a dry eye workup. With this dye, the anterior segment can be assessed using a cobalt blue filter; the examination can be further enhanced by using a Wratten #12 yellow filter (optional). The clinical use of fluorescein dye has two purposes: to evaluate tear film stability through TBUT and highlight epithelial defects. A TBUT of less than 10 seconds is considered abnormal and indicates tear film instability. Figure 4 demonstrates punctate staining as visualized with fluorescein dye.
Fluorescein can also aid in visualizing the tear meniscus; however, ideally the height is evaluated prior to instillation of any eye drops/dyes as the tear volume is altered and measurement will be overestimated.
A secondary dye (rose bengal or lissamine green) that stains devitalized cells should be incorporated into a dry eye evaluation as well. Both of these dyes are best used with white light in the slit lamp. These stains aid in detecting conjunctival staining, which can be present prior to corneal staining and can help detect more subtle DED.21
Vital dyes can also aid in visualizing lid wiper epitheliopathy (LWE). The lid wiper is an area of the marginal eyelid conjunctiva that is responsible for spreading the tear film across the ocular surface. Both upper and lower lids have a lid wiper area. Clinically, it is easiest to evaluate this area on the upper lid after performing lid eversion with lissamine green or rose bengal stain.
LWE occurs when the conjunctival epithelium in the lid wiper area is disrupted due to mechanical friction.22 LWE appears posterior to the line of Marx, which separates the epidermis of the eyelid and the conjunctival tissue. The line of Marx will variably stain with all dyes; it is important to use this as a reference point and exclude this staining from assessment for LWE (Figure 5).22 LWE is commonly seen in all types of dry eye but is especially prevalent in contact lens–associated DED.22
Supplementary quantitative tear film testing. If ADDE is suspected and a reduced tear meniscus is also noted, additional analysis of tear film production can be measured using Schirmer’s or phenol red threat tests. However, throughout the years, these tests are being used less and less due to variable diagnostic accuracy.10,11
 |
| Fig. 5. Slit lamp photo with the line of Marx visualized with lissamine green stain. Lid wiper epitheliopathy, when present, is visible posterior to the line of Marx. Click image to enlarge. |
Putting It All Together
By incorporating our knowledge of the etiological categories of DED with our clinical findings as related to both signs and symptoms, we are able to classify patients with ADDE, EDE or mixed-mechanism DED. Before making a management plan, it is important to rule out differential diagnosis, remembering that patients can have more than one condition and each condition requires proper management to reduce patient symptoms.
It is important to discuss with patients their risk factors and any iatrogenic causes of DED. Any modifiable risk factors should be reviewed in detail and addressed, if possible. As part of patient education, be sure to present any quantitative and qualitative data (e.g., InflammaDry, questionnaire, osmolarity, TBUT). These test results will vary (and hopefully improve) over time and can be a very meaningful and encouraging for patients to maintain their treatment protocol.
By understanding the pathophysiology of DED and following systematic evaluation techniques, DED diagnosis and management can become patient-centric and identify unsuspecting patients.
Dr. Gould is an assistant clinical professor at the SUNY College of Optometry University Eye Center and is responsible for clinical supervision of third- and fourth-year students in primary care. Dr. Gould receives consulting fees from Aerie Pharmaceuticals and Carl Zeiss Meditec.
| 1. Bartlett JD, Keith MS, Sudharshan L, et al. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;(9):1719-30. 2. Gomes JAP, Santo RM. The impact of dry eye disease treatment on patient satisfaction and quality of life: A review. Ocul Surf. 2019;1(17):9-19. 3. Craig JP, Nelson JD, Azar DT, et al. TFOS DEW II report executive summary. Ocul Surf. 2017;15(4): 802-12. 4. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and classification. Ocul Surf. 2017;15(3):276-83. 5. Mcmonnies CW. The potential role of neuropathic mechanisms in dry eye syndromes. J Optom. 2017;10(1):5-13. 6. Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404-37. 7. Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341-5. 8. Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res. 2013;117:79-87. 9. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea. 2012;31(5):472-8. 10. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-74. 11. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019; 45(5):669-84. 12. Stapleton F, Alves M, Bunya VY, et al. T FOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-65. 13. Gomez JAP, Azar DR, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-38. 14. Denoyer A, Landman E, Trinh L, et al. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122(4):669-76. 15. Hayirci E, Yagci A, Palamar M, et al. The effect of continuous positive airway pressure treatment for obstructive sleep apnea syndrome on the ocular surface. Cornea. 2012;31(6):604-8. 16. Nichols KK, Nichols JJ, Zadnik K. Frequency of dry eye diagnostic test procedures used in various modes of ophthalmic practice. Cornea. 2000;19(4):477-82. 17. Brissette AR, Drinkwater OJ, Bohm KJ, et al. The utility of a normal tear osmolarity test in patients presenting with dry eye disease like symptoms: A prospective analysis. Cont Lens Anterior Eye. 2019;42(2):185-9. 18. Scaffidi RC, Korb DR. Comparison of the efficacy of two lipid emulsion eyedrops in increasing tear film lipid layer thickness. Eye Contact Lens. 2007;33(1):38–44. 19. Kim AD, Muntz A, Lee J, et al. Therapeutic benefits of blinking exercises in dry eye disease. Cont Lens Anterior Eye. 2020;S1367-0484(20):30087-4. 20. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 21. Kim J, Foulks GN. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea. 1999;18(3):328-32. 22. Efron N, Brennan NA, Morgan PB, et al. Lid wiper epitheliopathy. Prog Retin Eye Res. 2016;53:140-74. |
