 |
Guidelines For IIH Management in Optometric Practice
Explore the causes and symptoms of this condition as well as effective management strategies.
By Theresa Jay, OD, Steve Njeru, OD, Rachel Werner, OD, and Melissa Chen, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: October 15, 2024
Expiration Date: October 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists interested in learning more about idiopathic intracranial hypertension, particularly management and treatment.
Educational Objectives: After completing this activity, participants should be better able to:
Recognize the underlying pathophysiology and risk factors of IIH.
Recognize its key systemic and ocular symptoms.
Identify the appropriate diagnostic criteria and procedures for patients with IIH.
Evaluate and recommend effective management and treatment options for this condition.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Drs. Jay, Njeru, Werner and Chen have no financial disclosures has no financial disclosures. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #129342 and course ID 93883-NO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
Idiopathic intracranial hypertension (IIH) is a systemic condition characterized by increased intracranial pressure without a known cause.1 It is a diagnosis of exclusion, meaning that life-threatening conditions must be ruled out before confirming IIH.1 Current hypotheses suggest that obesity is the main underlying risk factor, rather than gender or age alone, as this condition is strongly associated with women of reproductive age.1
The hallmark ocular finding in IIH is papilledema, which can be accompanied by symptoms such as new headaches, transient vision loss, double vision, photopsia and pulsatile tinnitus.1 This article will explore the pathophysiology, potential causes, systemic and ocular symptoms, diagnostic criteria, differential diagnoses, management and treatment for IIH.
Epidemiology and Pathophysiology
The incidence of IIH has been estimated to range from 0.03 to 7.8 per 100,000 people per year.2,3 The average age of diagnosis is between 23 and 36 years, with a higher prevalence among obese women compared with obese men.2,4 IIH is associated with metabolic conditions, such as obesity, increased weight gain and a history of polycystic ovary syndrome (PCOS).2 Recent trends suggest that the incidence of IIH may be rising, potentially due to the increasing prevalence of obesity.2 Patients with IIH face a higher risk of developing additional comorbidities, including a twofold increased risk of cardiovascular disease, a 30% increased risk of type 2 diabetes and a 55% increased risk of hypertension.2
The exact cause of IIH remains unclear, though it has been proposed that alterations in the dural venous sinus anatomy or cerebrospinal fluid (CSF) dysregulation might play a role. A study found dural venous stenosis in 27 of 29 patients with IIH, suggesting that such stenosis could lead to intracranial venous hypertension and increased intracranial pressure.5 Nevertheless, it is crucial to differentiate IIH from dural venous thrombosis, which can mimic IIH.5
Research has frequently focused on metabolic or hormonal factors due to IIH’s higher prevalence in obese women. It is hypothesized that an increase in adipose tissue may release more inflammatory mediators, such as leptin, which could enhance CSF secretion in the choroid plexus.2,6 Some studies also propose that an increase in central body fat might reduce CSF reabsorption by elevating central venous pressure. Another theory suggests that obese individuals might experience venous microthrombosis, leading to decreased CSF reabsorption.6 Although steroid hormones have been investigated, their role in IIH remains inconclusive.
 |
|
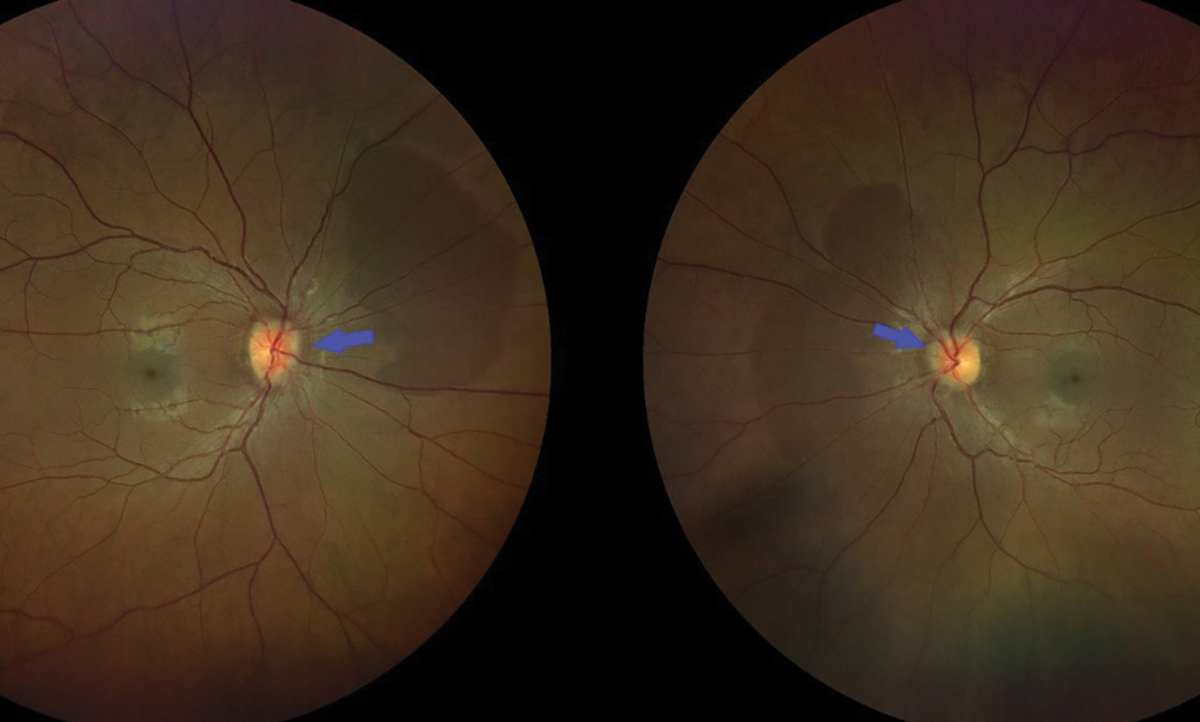
Fig. 1. Color fundus photo showing bilateral optic nerve head edema, characterized by indistinct margins which are indicated by the blue arrows. Click image to enlarge. |
Understand the Causes
This condition is idiopathic in nature, so neurological workups should reveal no identifiable cause for the increased intracranial pressure. However, secondary intracranial hypertension has been associated with certain systemic medications and conditions. Systemic medications linked to secondary intracranial hypertension include tetracyclines, retinoids, excessive vitamin A and hormonal medications, such as tamoxifen.2 It is recommended to discontinue these medications if IIH develops following their initiation.7 Systemic conditions associated with IIH include renal failure, anemia, Addison’s disease, Cushing’s disease and sleep apnea. In cases of sleep apnea, which predominantly affects men, nocturnal hypoxia is believed to lead to cerebral vasodilation and increased blood flow, ultimately raising intracranial pressure.
Presentation
IIH’s clinical presentation can vary, but prompt diagnosis is crucial to prevent permanent quality of life changes. Systemic symptoms often include headaches, nausea, vomiting and pulsatile tinnitus, which can be exacerbated by positions that increase intracranial pressure, such as lying supine, bending forward, coughing and Valsalva maneuvers.
Headaches are the most common systemic symptom in idiopathic intracranial hypertension, affecting a reported 75% to 94% of patients.8 These headaches are typically described as pressure-like, frontal and retro-orbital, often accompanied by a throbbing sensation. Nausea is reported in 72% to 75% of patients, while pulsatile tinnitus is present in a reported 52% to 60% of patients and usually bilaterally.8 This is a rhythmic swooshing or whooshing sound synchronized with the heartbeat.
 |
|
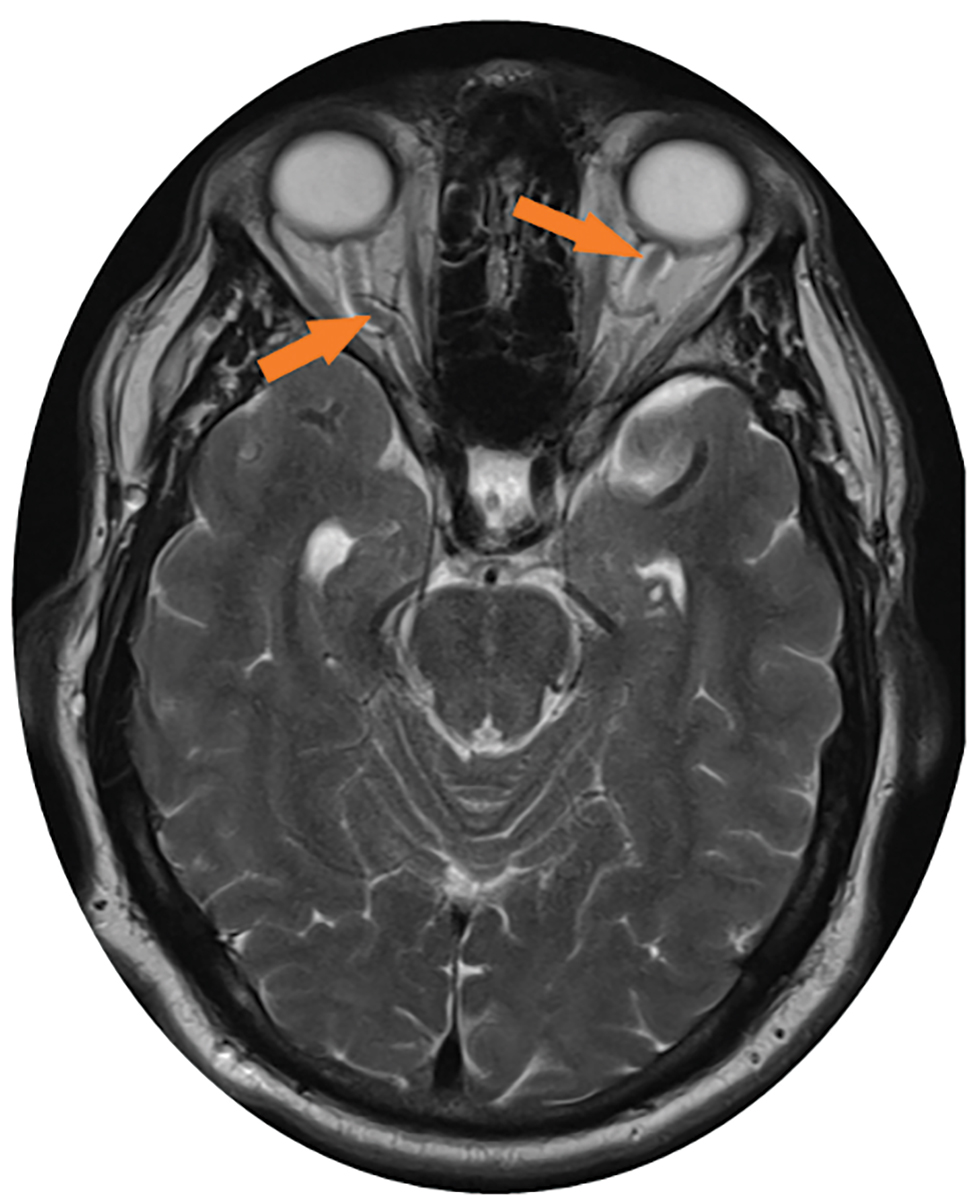
Fig. 2. This axial MRI of the brain without contrast shows the dilated (up to 8mm) and tortuous optic nerve sheaths, as indicated by the orange arrows. Click image to enlarge. |
Ocular symptoms in IIH can lead to significant visual impairment that affects daily activities of life. Patients may experience reduced visual acuity or visual field loss. Vision loss is often peripheral rather than central, unless the papillomacular region is affected. Transient vision loss can be unilateral or bilateral, lasting a few seconds to minutes, and is often described as blurry or foggy vision.
Over 70% of IIH patients experience visual field changes, including an enlarged blind spot, inferior nasal defects, partial arcuate defects and generalized field loss.9 Color vision loss, though less common, can occur in fewer than 20% of patients.2 Diplopia, or double vision, is also frequent due to a cranial nerve VI palsy. This palsy is thought to result from compression of the sixth nerve as it ascends from the pons and crosses the petrous portion of the temporal bone.9
A hallmark sign of IIH is papilledema, characterized by bilateral optic nerve head edema due to elevated intracranial pressure (Figure 1). The swelling occurs because increased CSF pressure disrupts the normal pressure gradient between intraocular pressure and retrolaminar pressure, leading to axoplasmic flow disturbances and neuronal flow stasis.2 This will ultimately result in optic nerve atrophy. Papilledema is typically symmetrical and is assessed using the Frisén scale, which ranges from stage 0 (normal optic disc) to stage 5 (severe papilledema).2 In cases of papilledema, a spontaneous venous pulse (SVP) is often absent due to increased intracranial pressure. Previous documentation of SVP can be valuable when diagnosing IIH, but its absence is not a definitive indicator of IIH, as some healthy eyes may also lack an SVP.
Diagnostic Criteria and Procedures
Diagnosing IIH can be challenging and typically requires multiple tests and imaging modalities for confirmation.10
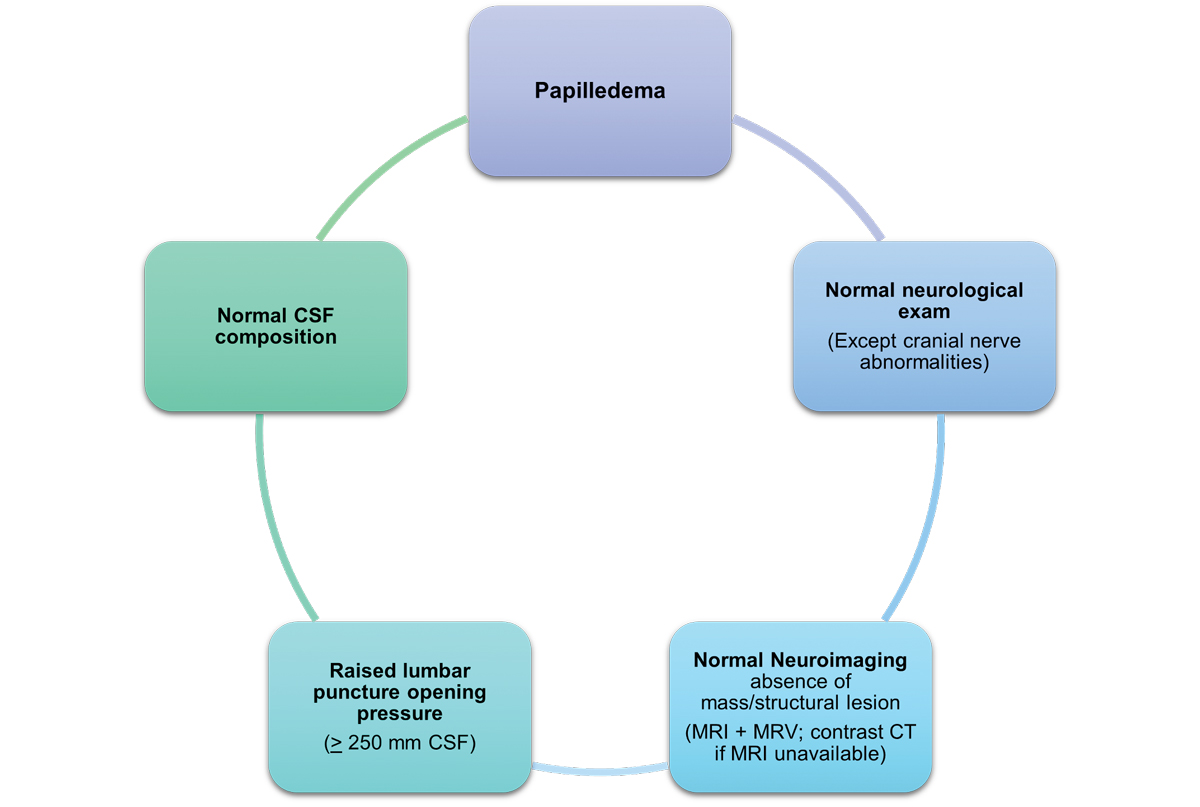
The criteria for diagnosing IIH include the following:11,12
- Papilledema.
- Normal neurologic exam except cranial nerve abnormalities.
- Normal brain parenchyma without evidence of hydrocephalus, mass or structural lesion and no abnormal meningeal enhancement on MRI/MRV with and without gadolinium contrast; contrast CT if MRI is contraindicated.
- Normal CSF composition.
- Elevated, properly performed lumbar puncture opening pressure.
- ≥250mm CSF (adults).
- ≥280mm CSF (children).
 |
|
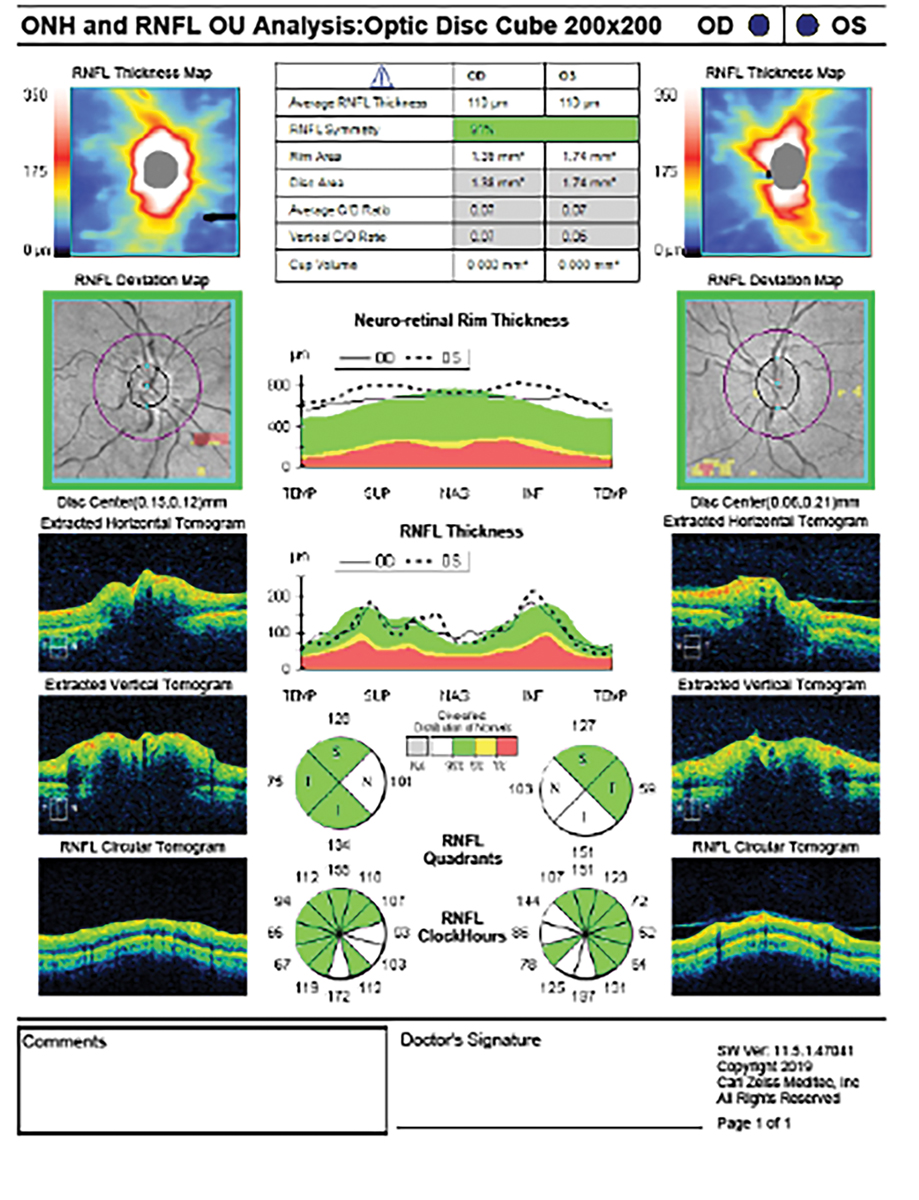
Fig. 3. The OCT-RNFL scan shows thickening of the nerve fiber layer in both eyes secondary to bilateral disc edema from IIH. This is evident on the RNFL thickness map, where a double rainbow sign indicates the areas of edema. Click image to enlarge. |
When papilledema is absent, the diagnosis of IIH requires meeting the diagnostic criteria, along with the presence of unilateral or bilateral abducens nerve palsy and at least three of the following findings.11,12
- Empty sella.
- Flattening of posterior aspect of globe.
- Distension of perioptic subarachnoid space with/without tortuous optic nerve (Figure 2).
- Transverse venous sinus stenosis.
Differential Diagnosis
If a patient is suspected of having IIH, a comprehensive ocular examination is crucial. This should start with fundoscopy to evaluate the optic nerve head and measuring blood pressure to rule out malignant hypertension before considering a referral for IIH.
The Frisén scale is used to categorize papilledema by grading the vascular appearance at the optic disc and the degree of disc margin blurring. Although this scale aims to standardize the assessment of papilledema, it can be significantly limited by intra- and inter-observer variability.13 Visual acuity assessment and visual field testing by perimeter are also essential.
Optical coherence tomography (OCT) is recommended for identifying, quantifying and monitoring papilledema. OCT captures optic nerve head edema and measures changes in the retinal nerve fiber layer (RNFL) and retinal pigment epithelium/Bruch’s membrane (RPE/BM) associated with acute and chronic changes in intracranial pressure (Figure 3).18
In IIH with papilledema, the RNFL becomes thicker due to axoplasmic flow stasis. This disruption in axoplasmic flow leads to the thickening of the RNFL, as it contains the axons of the swollen retinal ganglion cells (RGCs). This increase in thickness is often asymmetric, with more pronounced thickening in superior rather than inferotemporal areas of the nerve.14 The RNFL is generally thicker in true papilledema compared to pseudopapilledema, with 73% sensitivity and specificity. Additionally, pseudopapilledema affects only the inner peripapillary ring, whereas true papilledema impacts both the inner and outer rings.15
Additional helpful OCT nerve measurements help differentiate by evaluating the RPE/BM layer. In normal patients, the RPE/BM layer maintains a V-shape configuration that is angled away from the vitreous. In patients with papilledema due to IIH, this layer has been shown to invert, creating a U shape toward the vitreous.16,17
 |
|
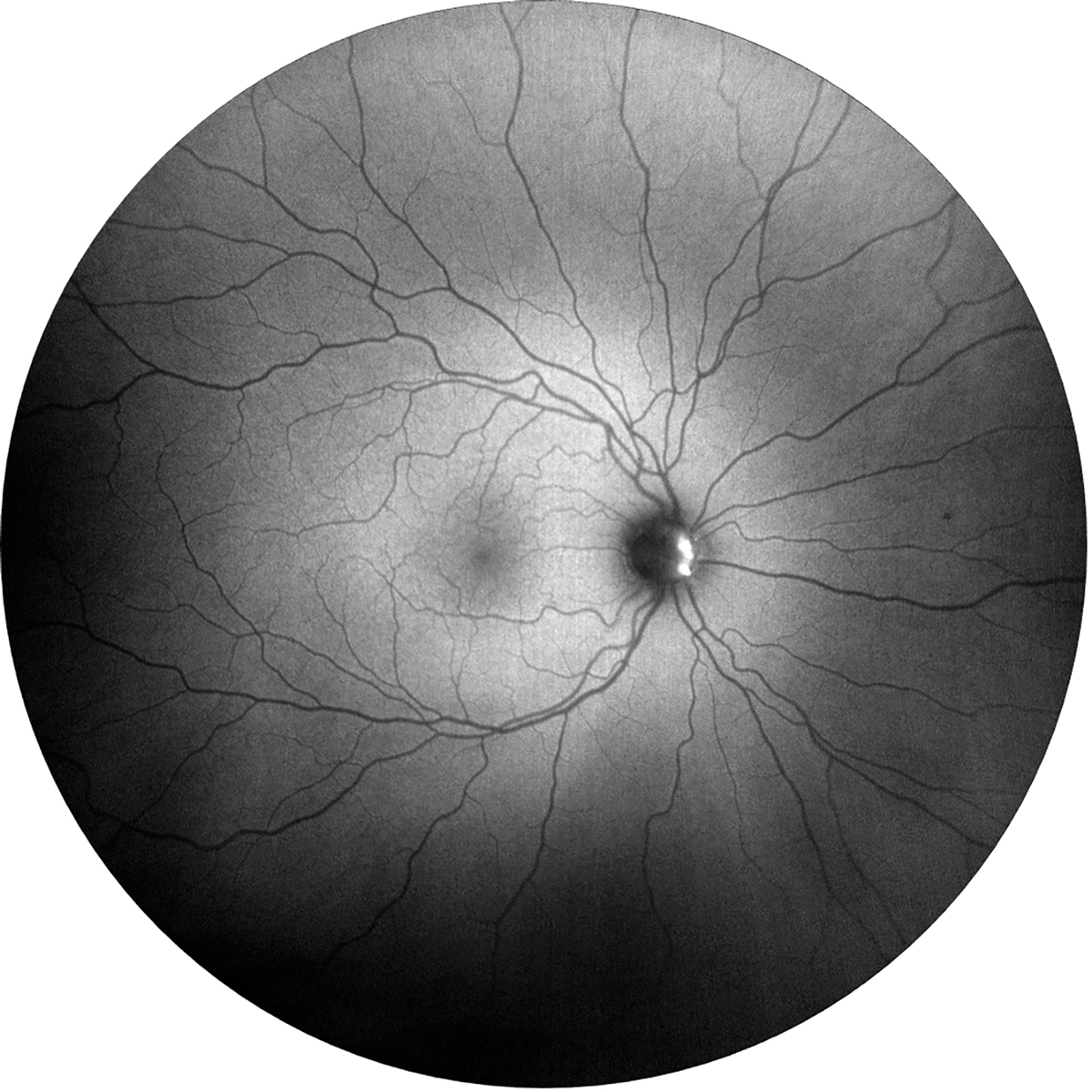
Fig. 4. Fundus autofluorescence photo showing hyperfluorescence within the nasal aspect of the optic nerve in the right eye due to optic nerve head drusen. Click image to enlarge. |
OCT angiography (OCT-A) can also be used by demonstrating tortuosity and dilation of large vessels as well as capillary tangling. This can help distinguish optic nerve damage with capillary dropout in anterior ischemic optic neuropathy.15 Fluorescein angiography may be used to help differentiate papilledema from pseudopapilledema. In papilledema, leakage occurs from the capillaries, followed by disc staining in later phases. Pseudopapilledema does not show early or late dye leakage from the capillaries at the disc.13
Fundus autofluorescence can help differentiate between disc drusen and papilledema. Both superficial and buried optic nerve drusen will appear as hyperfluorescent (Figure 4). It is important to note that disc edema can occur alongside disc drusen, so additional testing is warranted, as IIH is a diagnosis of exclusion.18 B-scan ultrasound measures optic disc height and the presence of excessive fluid within the optic nerve sheath, serving as a sensitive marker for increased intracranial pressure. This test is often used in combination with neuroimaging. Magnetic resonance imaging (MRI) can be used to detect the presence of an empty sella, which helps in specifying the diagnosis (Figures 5 and 6).14,15
Neuroimaging, preferably an MRI of the brain, is essential to rule out intracranial lesions. The main neuroimaging findings associated with IIH include an empty sella, flattening of the posterior aspect of the globe and enlargement of the perioptic subarachnoid space.12 Although some patients may also have cerebellar tonsillar ectopia, which can mimic a Chiari I malformation.19 MRI/CT venography may also be indicated to rule out smooth-walled narrowing of the transverse sinuses and sinus venous occlusions.16,17
Following neuroimaging, a lumbar puncture is necessary for fluid analysis and measuring opening pressure. Elevated opening pressure is defined as greater than 250mm H₂O in obese adults with a body mass index (BMI) greater than 30 and greater than 280mm H₂O in non-obese children.12,19 This procedure can be uncomfortable and may need to be repeated if results are atypical or minimally elevated.
 |
|
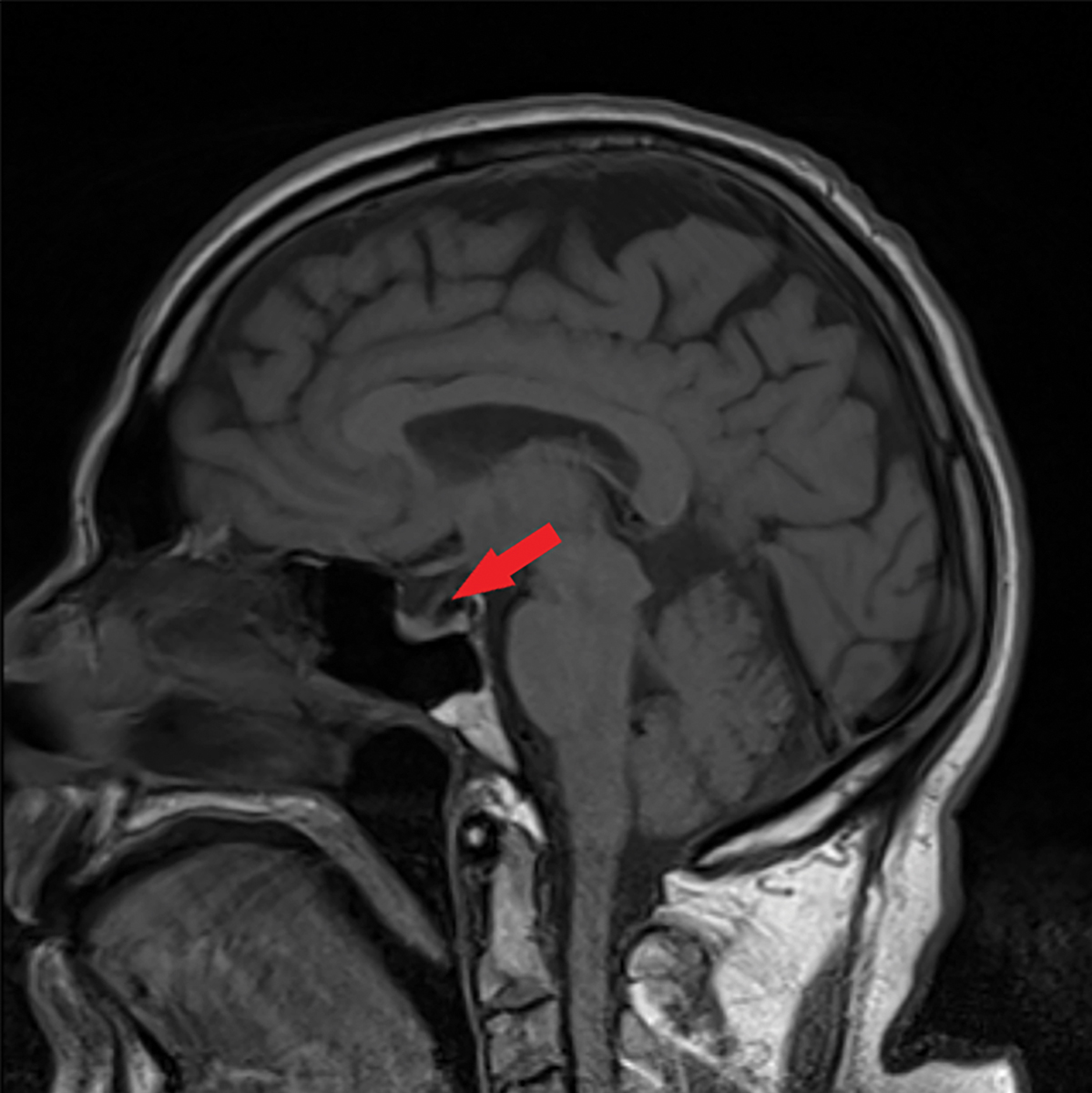
Fig. 5. This sagittal MRI of the brain without contrast shows a partially empty sella, as indicated by the red arrow. Click image to enlarge. |
When is IIH Not a Concern?
In this condition, retinal complications leading to visual impairment are less commonly discussed but are nonetheless significant. These retinal findings may include choroidal neovascularization, macular exudates, retinal or choroidal folds, venous stasis retinopathy and subretinal fluid. The development of choroidal neovascularization in IIH is not fully understood, but it is hypothesized that axoplasmic flow stasis, axonal swelling and poor vascular perfusion contribute to the formation of angiogenic factors.20-22 Choroidal folds, while not exclusive to papilledema, can be observed both at the onset and after the resolution of elevated intracranial pressure. These folds result from structural stress on the posterior globe and optic nerve due to the compressive forces of high intracranial pressure.22
Fortunately, macular exudates from capillary leakage generally have minimal impact on visual acuity. Venous stasis retinopathy is associated with poor venous outflow in a crowded optic nerve head.20 Subretinal fluid may be found as an extension from peripapillary fluid or a spread of optic disc edema.20,23 Although optic nerve findings are more prominent in IIH, recognizing these associated retinal abnormalities is valuable as they can also affect vision.
Exploring Treatment Options
The main goals for managing IIH are to address the underlying condition, protect vision and reduce headache morbidity. Weight loss is a primary management option, though it can be challenging to achieve and maintain. It remains the first-line recommendation before considering medication and/or surgery. Evidence shows that weight loss can lower intracranial pressure, alleviate symptoms and reduce RNFL thickness.2 However, the exact amount of weight loss needed to ensure remission is not yet well defined and requires further research.
 |
|
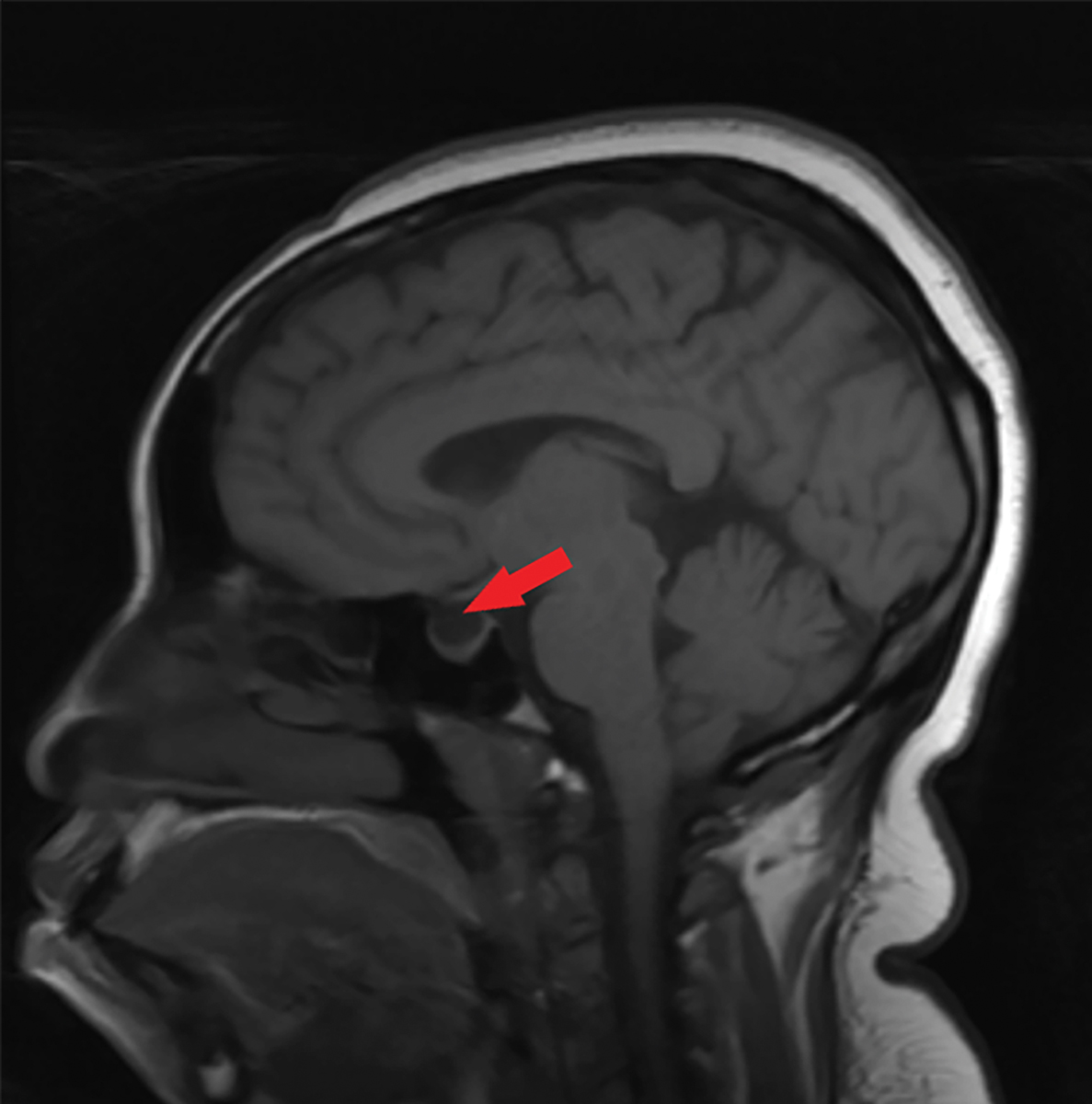
Fig. 6. This sagittal T1 weighted MRI of the brain without contrast shows an empty sella, as indicated by the red arrow. Click image to enlarge. |
Acetazolamide, a carbonic anhydrase inhibitor, is commonly used to manage IIH while patients work on weight loss. It decreases cerebrospinal fluid production by the choroid plexus.2 Although two randomized studies have demonstrated its efficacy in improving visual field mean deviation, papilledema grading and vision-related quality of life, there were no significant differences in visual acuity or headache disability scores.2 Furthermore, 48% of patients in one study could not tolerate acetazolamide due to side effects like nausea, vomiting, diarrhea and fatigue.2
Topiramate, an anticonvulsant with appetite-suppressing effects, is another medication option but not commonly used in cases of IIH. A study comparing topiramate with acetazolamide found no significant intergroup differences as both treatment groups showed visual field improvements over 12 months.2 However, the topiramate group experienced significantly greater weight loss due to its appetite-suppressing properties.2
For cases of rapid vision loss or when medical treatments are intolerable, surgical intervention is necessary. Surgical options include CSF diversion, venous sinus stenting and optic nerve sheath fenestration. This last option involves creating slits in the optic nerve sheath to lower CSF pressure in the subarachnoid space surrounding the optic nerve head. It effectively reduces papilledema and improves visual fields but may lead to complications such as diplopia and anisocoria.2 Approximately 16.9% of patients may need additional procedures like CSF diversion or VSS for headache relief.2
CSF diversion procedures, such as a lumboperitoneal or ventriculoperitoneal shunt, lower intracranial pressure and alleviate headaches and papilledema. However, these procedures have a 12-month failure rate of roughly 39%, with patients averaging 2.6 shunt revisions.2 Potential complications include CSF leak, tonsillar herniation and intracerebral hemorrhage.2
 |
|
Fig. 7. This image highlights the diagnostic criteria that can diagnose IIH in the presence of papilledema.7 Click image to enlarge. |
Venous sinus stenting is a newer surgical approach that reduces venous hypertension and improves CSF drainage by relieving stenotic segments of the transverse sinus. It also decreases papilledema, enhances visual fields and reduces headache symptoms. The 12-month failure rate for this option is approximately 13.1%, with complications that may include subdural, subarachnoid and intracerebral hemorrhage or obstructive hydrocephalus.2
Takeaways
IIH should be treated as a medical emergency until proven otherwise. Optometrists play a crucial role in its diagnosis, as ocular findings may present before systemic symptoms. Key ocular signs to monitor include papilledema, blurred vision, changes in the visual field and double vision, which may indicate a cranial nerve VI palsy. IIH can significantly impact visual function and quality of life, with papilledema potentially causing permanent changes in visual acuity and visual field, while chronic headaches can be debilitating and challenging to manage.
This condition is most commonly associated with obesity and is more prevalent in women of reproductive age. However, it can also be linked to systemic medication use and other metabolic or hormonal factors, which requires a thorough review of the patient’s medical history. Diagnosing IIH requires an MRI and lumbar puncture to exclude lesions and assess intracranial pressure and cerebrospinal fluid composition (Figure 7). Given this condition is a diagnosis of exclusion, other potential pathologies must be ruled out first.
The primary management strategy for IIH involves weight loss, which may be complemented by medical therapies such as acetazolamide. Surgical interventions, including CSF diversion, venous sinus stenting and optic nerve sheath fenestration, are reserved for severe cases, particularly those involving rapid vision loss.
As primary eyecare providers, optometrists are on the frontlines of healthcare and, therefore, must be prepared to diagnose and manage IIH, paving the way for optimal patient outcomes and quality of life.
Dr. Jay is an active staff optometrist at the Kernersville VA Health Care Center. She received her Doctor of Optometry from The Ohio State University College of Optometry in 2020. She then went on to complete her ocular disease/primary eye care residency at the W.G. (Bill) Hefner VA Medical Center. She serves as vice president of the North Carolina Chapter of American Academy of Optometry (AAO).
Dr. Njeru is a staff optometrist at the Chillicothe VA Medical Center. He graduated from The Ohio State University: College of Optometry and completed his ocular disease residency at the Chillicothe and Columbus VA. He is a fellow of the AAO.
Dr. Werner is a staff optometrist at the Kernersville VA Health Care Center. She received her Doctor of Optometry from Southern College of Optometry in 2020. She then went on to complete her Low Vision/Primary Care residency at the Jamaica Plain VA Medical Center. She is a fellow of the AAO and serves as treasurer of the North Carolina Chapter of AAO.
Dr. Chen is a staff optometrist at the Charlotte VA Health Care Center. She received her Doctor of Optometry from Salus University, Pennsylvania College of Optometry in 2019. She then went on to complete her residency at the W.G. (Bill) Hefner VA Medical Center. She also serves as an examiner at the National Board of Examiners in Optometry and as secretary of the North Carolina Chapter of AAO. They have no disclosures.
1. Mondragon J, Klovenski V. Pseudotumor cerebri. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. www.ncbi.nlm.nih.gov/books/NBK536924/. Updated September 29, 2022. Accessed. September 16, 2024. 2. Wang MTM, Bhatti MT, Danesh-Meyer HV. Idiopathic intracranial hypertension: pathophysiology, diagnosis and management. J Clin Neurosci. 2022;95:172-9. 3. Malm J, Kristensen B, Markgren P, Ekstedt J. CSF hydrodynamics in idiopathic intracranial hypertension: a long-term study. Neurology. 1992;42(4):851-8. 4. Radhakrishnan K, Ahlskog JE, Cross SA, et al. Idiopathic intracranial hypertension (pseudotumor cerebri). Descriptive epidemiology in Rochester, MN, 1976 to 1990. Arch Neurol. 1993;50(1):78-80. 5. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60(9):1418-24. 6. Toscano S, Lo Fermo S, Reggio E, et al. An update on idiopathic intracranial hypertension in adults: a look at pathophysiology, diagnostic approach and management. J Neurol. 2021;268(9):3249-68. 7. Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005;6(1):29-37. 8. Markey KA, Mollan SP, Jensen RH, et al. Understanding idiopathic intracranial hypertension: mechanisms, management and future directions. Lancet Neurol. 2016;15(1):78-91. 9. Raoof N, Hoffmann J. Diagnosis and treatment of idiopathic intracranial hypertension. Cephalalgia. 2021;41(4):472-8. 10. Hoffmann J, Mollan SP, Paemeleire K, et al. European headache federation guideline on idiopathic intracranial hypertension. J Headache Pain. 2018;19(1):93. 11. Friedman DI. The pseudotumor cerebri syndrome. Neurol Clin. 2014;32(2):363-96. 12. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159-65. 13. Frisén L. Swelling of the optic nerve head: a backstage view of a staging scheme. J Neuroophthalmol. 2017;37(1):3-6. 14. Ophir A, Karatas M, Ramirez JA, Inzelberg R. OCT and chronic papilledema. Ophthalmology. 2005;112(12):2238. 15. Fard MA, Fakhree S, Abdi P, et al. Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol. 2014;158(1):136-43. 16. Sibony PA, Kupersmith MJ. Geometric morphometrics of the peripapillary RPE layer by OCT. Invest Ophthalmol Vis Sci. 2011;52(14):3004. 17. Kupersmith MJ, Sibony P, Mandel G, et al. Optical coherence tomography of the swollen optic nerve head: Deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52(9):6558-64. 18. Skau M, Milea D, Sander B, et al. OCT for optic disc evaluation in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2011;249(5):723-30. 19. Aiken AH, Hoots JA, Saindane AM, Hudgins PA. Incidence of cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. AJNR Am J Neuroradiol. 2012;33(10):1901-6. 20. Nichani P, Micieli JA. Retinal manifestations of idiopathic intracranial hypertension. Ophthalmol Retina. 2021;5(5):429-37. 21. Pereira A, Nichani PAH, Yan P, Micieli JA. Subfoveal choroidal neovascular membrane secondary to idiopathic intracranial hypertension. J Vitreoretin Dis. 2024;8(2):192-5. 22. Ariello LE, Mello LGM, Pimentel SLG, Monteiro MLR. Chorioretinal abnormalities in idiopathic intracranial hypertension: case reports. Int J Retina Vitreous. 2022;8(1):48. 23. Schirmer CM, Hedges TR 3rd. Mechanisms of visual loss in papilledema. Neurosurg Focus. 2007;23(5):E5. |
