Going Antiviral: How to Bring Herpes to a Halt
Here’s a review of the most commonly prescribed topical and oral antiviral medications used to manage herpetic eye disease.
Goal Statement:
The visual consequence of ocular disease can be painful, devastating and life-long if not treated both promptly and aggressively. The purpose of this article is not to focus on the different presentations of HSV and VZV, but to describe the antiviral medications currently available. It will also offer a glimpse into the future of herpes infection prevention.
Faculty/Editorial Board:
Dr. Michael J. Lyons is a staff optometrist at the Uveitis and Cornea Clinic at the Cincinnati Eye Institute and a volunteer faculty member at the University of Cincinnati. He’s also the founder of Focal Pointe Eye Care, a private, full-service optometric office in West Chester, Ohio.
Credit Statement:
This course is COPE approved for 2 hours of CE credit. COPE ID is 44093-AS. Please check your state licensing board to see if this approval counts toward your CE requirement for relicensure.
Joint-Sponsorship Statement:
This continuing education course is joint-sponsored by the Pennsylvania College of Optometry.
Disclosure Statement:
Dr. Lyons has no relationships to disclose.
It can be difficult and awkward to talk about herpes. Yet, humans are the only natural host reservoir for both the herpes simplex virus (HSV) and the varicella zoster virus (VZV). Studies have indicated that adults may test as high as 90% for HSV antibodies and 95% for VZV antibodies, suggesting a history of prior infections.1,2
So, maybe instead of being reluctant to talk about herpes, we should think about it and ask patients about it more often.
Viruses are the smallest of infectious pathogens, with the herpes organism ranging from 120nm to 300nm in size. Although tremendously small, viruses demonstrate extraordinary latency and patience. Herpes possesses the unique characteristic of incorporating its viral genome into the host’s deoxyribonucleic acid (DNA). This process renders the virus undetectable by the human immune system while allowing the host cell to survive.3 Thus, the viruses can remain dormant for decades before being reactivated by changes in the host’s immune system, stress or other environmental factors.
The spectrum of ocular disease that is caused by the herpes family varies widely––from blepharitis at the ocular adnexa all the way to retinitis in the posterior segment. One study published in 1980 indicated that the specific strain of herpes involved, as well as a variety of ancillary host factors, can yield different clinical presentations of herpetic ocular disease.4 Because of this, the diagnosis and treatment of ocular herpetic disease becomes a challenge––especially in the case of herpes simplex, when the condition does not exhibit traditional findings.
The purpose of this article is not to focus on the different presentations of HSV and VZV, but to describe the antiviral medications currently available. It will also offer a glimpse into the future of herpes infection prevention.
Replication Cycle of Herpes
Opinions vary on whether viruses are actually a form of life. Unlike most living organisms, viruses have no cellular structure or metabolism and are unable to self-replicate. However, they do possess DNA and have the ability to evolve through natural selection, making them more “life-like.”5
Both simplex and zoster are similar in structure, consisting of a double-stranded DNA surrounded by an icosahedral capsid, with an outer cell membrane containing glycoproteins, carbohydrates and lipids. Because they are acellular, viruses depend on host cells to provide the structure and metabolism necessary for replication.
The primary virus infection starts when it binds to and invades a host cell and then initiates its own DNA replication. After the new viral components are made, the host cell releases new viruses that can infect other host cells. This type of infection produces an acute herpetic outbreak that is usually limited by the response of the host’s immune system.6
The replication cycle of the herpes virus can be summarized into six stages:
1. Attachment. Using specialized receptors, the viral cell attaches to the host cell.
2. Penetration. The virus enters the host cell.
3. Uncoating. This is the process of the capsid being removed, releasing the DNA into the host cell.
4. Replication. The host’s DNA is used to facilitate viral DNA proliferation.
5. Assembly. New viruses are formed within the host cell.
6. Release. Viruses exit the host cell and often experience lysis, resulting in cell death.
Additionally, some viruses enter a dormant state and will not immediately proceed through the entire replication cycle. Viral latency can occur within weeks of the primary infection, halting the replication of viral DNA inside the host.7 This potential outcome is further characterized by circularization of the viral genome, with only very limited gene expression.8 HSV latency usually takes place in the trigeminal ganglion, and VZV latency generally occurs in the sensory spinal or cerebral ganglia.6,8
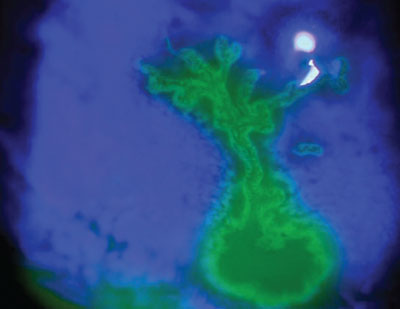 |
| Fluorescein staining of a herpes simplex keratitis dendrite. |
Treatment Considerations
The development of effective antiviral agents has been much slower than the development of antibacterial drugs. This is attributed to the behavior of the virus following attachment to the host. Viruses are dependent on the host for growth and replication, so once the virus enters the host cell, it becomes part of the cell’s metabolism, making it harder to eradicate.
It is important to note that all antiviral medications are at least somewhat toxic to the host cells. The challenge of creating an effective antiviral medication is making it selective for the virus while not disrupting the host cell too significantly. The agent’s ability to perform within these parameters ultimately determines the clinical usefulness of the drug. Most of the antiviral drugs available today are antimetabolites, which disrupt DNA synthesis, halt further replication and render the virus incapable of infecting new hosts.6
The following antiviral medications are listed below in order of development:
Topical agents:
• Idoxuridine. In the early 1950s, idoxuridine became the first available topical antiviral medication. It was initially developed as an anticancer drug.9
Idoxuridine is a thymidine analog that inhibits DNA polymerases, preventing the incorporation of thymidine into viral DNA.10 The agent is highly toxic, systemically and topically, due to its unselective pairing with both host and viral DNA.
Today, idoxuridine is available as a 0.1% solution and 0.5% ointment for topical ocular use, and is available through compounding pharmacies. The drug is effective against HSV only.
• Vidarabine. Like idoxuridine, vidarabine was initially developed as an anticancer drug in 1960.11 The agent also inhibits viral DNA polymerase, but this is due to its similarity to adenosine. Vidarabine is phosphorylated by both host and viral kinases, but is a more potent inhibitor of viral DNA polymerase than host DNA polymerase.6 This makes the agent safe to use systemically, because it is less toxic to the host cell than idoxuridine.
Vidarabine was once available in the US as a 3% ointment (Vira-A, Monarch Pharmaceuticals), but was discontinued. It is still available on a limited basis as a compounded ointment. It is effective against both HSV and VZV. Vidarabine generally is less potent than other available agents, but is still useful in cases of suspected antiviral resistance.10
• Trifluridine. This thymidine analog also was originally developed as an anticancer agent.12 Studies have indicated that trifluridine is superior in antiviral efficacy to both idoxuridine and vidarabine, making it the preferred treatment for infectious epithelial keratitis.10 But, trifluridine is still toxic to the host cells.
The agent is available as a 1% ocular solution (Viroptic, Monarch Pharmaceuticals), and is effective against HSV.
Growing Concerns of Acyclovir ResistanceHSV’s ever-increasing resistance to antiviral agents is of fundamental concern to both pharmaceutical researchers and health care providers. Acyclovir resistance has increased with the expanded use of antiviral therapy. Resistance most commonly occurs in immunocompromised patients with chronic and/or progressive infections who’ve been subjected to prolonged or repeated courses of therapy.16 In such patients, an impaired immune system cannot fully suppress viral replication. Hence, the remaining medication load is the only means of antiviral activity. A study published in 2008 showed that 6.4% of viral isolates found in 173 immunocompromised patients with herpetic keratitis were resistant to acyclovir.34 In general, antiviral resistance should be suspected if the clinical response to therapy is less than that anticipated on the basis of prior experience.35 |
• Acyclovir. Unlike the aforementioned antiviral agents, acyclovir is highly selective and thus far less toxic. Its commercial release in 1982 marked a profound shift in the development of future antiviral medications.
Acyclovir is guanosine analog, and like other antiviral agents, it requires phosphorylation in order to become activated. However, acyclovir is only phosphorylated by virus-infected host cells containing viral thymidine kinase, making this significantly more toxic to viruses than the host.10 Once phosphorylation takes place, it selectively inhibits viral DNA synthesis by binding to DNA polymerase, resulting in chain termination.13
Acyclovir is active against both HSV and VZV. It is available in Canada and Europe as a 3% ointment, but can be compounded at select pharmacies in the US. It also is available as an oral agent.
• Bromodeoxyuridine. This agent’s mode of action is similar to that of acyclovir. Bromodeoxyuridine is commercially available in Europe, but not in the US. It is important to note that the drug has been shown to cause liver toxicity.14
• Ganciclovir. Initially approved for intravenous treatment of cytomegalovirus retinitis in AIDS patients, ganciclovir is now available as a 0.15% gel (Zirgan, Bausch + Lomb). The agent also selectively inhibits DNA polymerase in only virus-infected cells.
A study published in 1997 showed that ganciclovir gel’s therapeutic efficacy was comparable to that of 3% acyclovir ointment, but patients tolerated ganciclovir gel much better.15
Oral Agents:
• Acyclovir. As previously noted, acyclovir has become the prototype of antiviral agents due its selective nature against virus-infected cells. It is the most frequently prescribed oral antiviral agent in the United States, and has been commercially available for more than two decades.
It has demonstrated remarkable safety and efficacy against HSV and VZV in both normal and immunocompromised patients.16 However, the bioavailability of acyclovir is poor, with just 15% to 30% of the oral formulation being absorbed.17 In order to achieve better serum concentrations, higher doses of oral acyclovir are required.
Acyclovir is available in 200mg capsules and 400mg or 800mg tablets (Zovirax, GlaxoSmithKline). It is also available in a 200mg/5ml suspension.
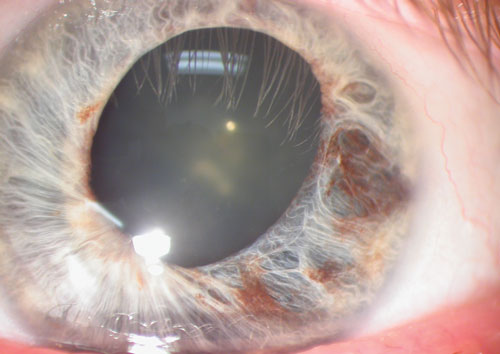 |
| This herpes simplex keratitis patient exhibited diffuse iris atrophy. |
• Famciclovir. Made available in 1994, famciclovir is a prodrug of penciclovir. Once famciclovir is absorbed, it is rapidly converted to penciclovir by viral thymidine kinase found in virus-infected cells.
Similar to acyclovir, famiciclovir inhibits DNA polymerase by competing with guanosine during viral replication. However, the agent is approximately 100-fold less potent than acyclovir in inhibiting herpes virus DNA polymerase activity.16 But because of its 65% to 77% bioavailability and long plasma half-life, it remains an effective antiviral agent.3
Famciclovir is active against HSV and VZV, and is available in 125mg, 250mg or 500mg tablets.
• Valacyclovir. Again, acyclovir’s primary weakness is its poor bioavailability. Valacyclovir is a prodrug that converts to acyclovir, which yields the same mechanism of action, antiviral spectrum and resistance profiles as those of its parent drug.16 Following oral administration, valacyclovir is hydrolyzed by esterases in the gastrointestinal tract and liver, converting to acyclovir. This provides a bioavailability exceeding 50%, which is three to five times greater than that of oral acyclovir.18 It is available in 500mg and 1,000mg tablets.
Herpes Simplex Virus
HSV is classified as either type 1 (HSV-1) or type 2 (HSV-2). Although HSV-1 usually involves the oropharynx and HSV-2 usually involves the genital area, both types have been shown to infect both areas.19 For the purpose of this review, we will focus on HSV-1, because it typically is the root cause of associated ocular disease. (An exception is herpes keratitis in neonates, which is caused by HSV-2 in up to 75% of cases.20 )
There are an estimated 20,000 new cases of ocular HSV in the United States per year, and more than 48,000 episodes reported annually.21 For HSV to cause an ocular infection, it first must enter the body through mucous membranes or the skin. The oral route is the most common pathway, as it provides access to the trigeminal ganglion, which ultimately leads to ocular infection. Interestingly, the primary HSV infection upon viral entry may be asymptomatic, with just 1% to 6% of infected individuals exhibiting clinical manifestations.10
After entry into the host, and primary infection with viral replication within the oral mucosa, HSV travels in a retrograde fashion to the trigeminal ganglion via the maxillary (V2) or mandibular (V3) branch of the trigeminal nerve (CN V). There, the virus resides in a latent state during the host’s lifetime.
During a period of systemic and/or immunologic stress, viral replication starts again in the ganglion, resulting in recurrent disease to the end organ. However, recurrent disease does not have to follow the same path. In the case of ocular disease, for example, HSV travels via the ophthalmic nerve (V1).
Many factors have been implicated in the reactivation of latent HSV, including sunlight exposure, trauma, heat, abnormal body temperature, menstruation, emotional stress and the presence of other infections.10
Clinical manifestations of HSV include blepharitis, conjunctivitis, iridocyclitis and keratitis. HSV keratitis may be classified into following groups and subgroups:10
I. Infectious epithelial keratitis.
a. Corneal vesicles.
b. Dendritic ulcer.
c. Geographic ulcer.
d. Marginal ulcer.
II. Neurotophic keratopathy.
III. Stromal keratitis.
a. Necrotizing stromal keratitis.
b. Immune stromal (interstitial) keratitis.
IV. Endotheliitis.
a. Disciform.
b. Diffuse.
c. Linear.
HEDS Gives Prophylactic Treatment a NodThe HEDS study was a set of multicenter, randomized, placebo-controlled trials sponsored by the National Eye Institute. They were designed to explore some of the questions associated with the treatment of HSV. Findings relative to antiviral treatment included: • There was no statistically or clinically significant benefit in using oral acyclovir for the treatment of HSV stromal keratitis in patients who received concomitant topical cortico-steroids and trifluridine with regard to time to treatment failure, proportion of patients who failed treatment, number of patients who experienced resolution, time to resolution or six-month best-corrected visual acuity.23 • While the number of patients recruited in this trial was too small to achieve statistically conclusive results, patient outcomes suggest a benefit of oral acyclovir in the treatment of HSV iridocyclitis in those receiving topical corticosteroids and trifluridine prophylaxis.36 • After ocular HSV resolution within one year, 12 months of treatment with oral acyclovir reduces the rate of recurrent ocular and orofacial HSV disease. Long-term antiviral prophylaxis is most important for patients with a history of HSV stromal keratitis, because it can prevent additional episodes and potential loss of vision.37,38 The HEDS illustrated the importance of oral antiviral medications in the treatment of ocular HSV disease. However, the study did not account for individualized treatment considerations and included just 12 months of follow-up data. It is important to note that a separate research team showed that long-term oral acyclovir use seems to effectively decrease the number of ocular HSV recurrences beyond 12 months.39 Thus, it appears that some strains of ocular HSV disease may benefit from long-term, if not life-long, prophylactic treatment.39 |
Recurrent HSV Disease
Considering the nature of ocular HSV, recurrent disease is a significant issue that can lead to devastating visual impairment and life-long treatment. In 1989, one research team evaluated 294 episodes of ocular HSV infection and reported recurrence rates of 9.6% at one year, 22.9% at two years and 63.2% at 20 years. After a second episode, 70% to 80% of patients had another recurrence within 10 years.22
Additionally, the Herpes Eye Disease Study (HEDS) followed 346 patients who were diagnosed with ocular HSV within the previous year. The HEDS researchers documented a recurrence rate of 18% for both epithelial keratitis and stromal keratitis during the 18-month study period (see “HEDS Gives Prophylactic Treatment a Nod.").
Antiviral Dosing and Recommendations
Ocular HSV, especially when recurrent, is a complex condition that doesn’t always follow a set of rules. When talking with colleagues from other practices, I have also found that treatment strategies vary widely. An example of this is the use of oral vs. topical antiviral medications in the treatment of HSV epithelial keratitis.
One study, for instance, showed that oral acyclovir may be as effective as topical acyclovir, and thus some practices prefer this approach.23 However, I believe that as long as you have a consistent plan in place and keep an open mind, you will be successful in disease control.
Topical antiviral dosage recommendations:
• Idoxuridine
- One drop 0.1% solution every hour while awake.
- Apply 0.5% ointment five times per day.
• Vidarabine ointment
- Apply five times per day until ulcer is resolved.
• Trifluridine
- One drop nine times per day.
• Acyclovir ointment
- Apply five times per day.
• Bromovinyldeoxyeridine
- Not commercially available in United States.
• Ganciclovir gel
- One drop five times per day until ulcer heals, then one drop three times per day for seven days.
Because herpetic infections heal at different rates, you may need to modify the aforementioned dosing regimens. Also, be sure to discontinue the topical antiviral medication once the ulcer is healed in order to avoid epithelial toxicity.
Oral antiviral dosing recommendations:
• Acyclovir
- Active: 200mg to 400mg five times daily.
- Suppression: 400mg to 800mg twice daily.
• Famciclovir
- Active: 250mg three times daily.
- Suppression: 125mg to 250mg twice daily.
• Valacyclovir
- Active: 1,000mg to 3,000mg daily.
- Suppression: 500mg 1,000mg daily.
Varicella Zoster Virus
As we know, varicella-zoster virus (VZV) causes two distinct clinical conditions: varicella (chickenpox) and herpes zoster (shingles). It is important to distinguish varicella as the primary infection and herpes zoster as the reactivation of latent VZV within the sensory spinal or cerebral ganglia.
Serological studies indicate that 95% of the population within the United States has evidence of prior VZV infections.2 The varicella vaccination was introduced in the United States in 1995. Prior to the vaccination, however, approximately four million cases of VZV infection occurred annually in the US, with the peak age of varicella occurrence at five to nine years.2 In fact, before widespread use of the vaccination, more than 90% of American children had been infected with varicella before age 15.2 Today, however, the incidence of varicella in the US has since declined by 57% to 90%.8
What to Know About HZOHerpes zoster ophthalmicus (HZO) results from herpes zoster involvement in the ophthalmic division of the trigeminal nerve, and can cause eyelid edema, conjunctivitis, episcleritis, scleritis, keratitis and uveitis. If vesicles present at the side or tip of the nose (i.e., Hutchinson’s sign) the patient’s risk of ocular involvement is approximately 50% to 76%.9 If Hutchinson’s sign is absent, however, the risk decreases to just 34%.8 The guidelines listed below are appropriate for short-term treatment of acute herpes zoster ophthalmicus: • Acyclovir: 800mg five times daily for seven to 10 days. Keep in mind, however, that the complexity of HZO may necessitate the use of both oral and topical antiviral therapy over an extended period. |
VZV Reactivation
The risk of developing herpes zoster is 10% to 30% in the US.24 Prior to the introduction of the varicella vaccination, the incidence of herpes zoster ranged from 1.2 to 6.5 cases per 1,000 individuals––with approximately 500,000 cases reported annually in the United States.25 Rates are gradually increasing among adults in the United States, but no correlation has been found between its increased incidence and the advent of the varicella vaccination.26
Upon initial infection with varicella, VZV is transported from the vesicular lesions by the sensory axons, where it ultimately enters a state of latency in the dorsal roots or trigeminal ganglia. Reactivation of VZV involves an alteration of the immune system in association with age, truma and/or aneural degeneration.8 Once reactivated, the virus replicates within the various ganglia and then travel via axonal transport, resulting in the characteristic unilateral dermatomal eruption of herpes zoster. Cranial nerve involvement occurs in 13% to 20% of all cases, with the trigeminal nerve seen most frequently.27
Currently Available Vaccinations
• Varivax (Merck), a live attenuated vaccine for varicella, received FDA approval in 1995 and was swiftly incorporated into the recommended immunization schedule for children. The vaccine was recommended for any infants, children, adolescents and adults in the US without a history of chickenpox (and without concurrent pregnancy).8
After nearly two decades of varicella vaccinations, disease incidence has been reduced by 57% to 90%.28-30 Despite these impressive statistics, controversy still surrounds continued use of the vaccine. In 2002, one research team speculated that the use of vaccinations may lead to an increase of adult-onset varicella and a greater incidence of herpes zoster.31 The researchers contended that intermittent exposure to those with chickenpox might boost immunity levels to both chickenpox and herpes zoster.31
Another research group predicted that there will be an increase in the incidence of herpes zoster over the next five to 40 years, but after that, the overall risk of herpes will decline progressively.32
• Zostavax (Merck) was approved in 2006 as an immunization booster for the prevention of herpes zoster in immunocompetent individuals 60 years and older with no prior history of herpes. It is not indicated in those with prior herpes zoster, because an outbreak naturally boosts the immunity. Zostavax is made of the same modified virus as Varivax, but given at a higher dosage.
The Shingles Prevention Study reported an overall herpes zoster incidence reduction of 51%, a reduced burden of illness zoster by 61% and a reduced incidence of postherpetic neuralgia by 66%.33 The duration of the vaccine’s protective effect is unknown, and currently there is no recommendation for a booster vaccination.8
The presentation of herpetic ocular disease is highly variable––the available antiviral treatment, however, is not. The visual consequence of ocular disease can be painful, devastating and life-long if not treated both promptly and aggressively. The use of antiviral medications plays an important role in disease control.
During the creation of this article, I encountered a monocular patient with a best-corrected visual acuity of 20/200 who manifested extensive ocular surface disease from an old traumatic injury. His chief complaint was a further visual decrease in his only functioning eye. The presentation was far from typical, with severe surface inflammation and corneal edema, but no ulceration or intraocular inflammation.
My initial thought was severe stem cell disease with neurotrophic keratopathy—but then I asked myself: “Could it be herpes?”
On follow-up several days later, a disciform appearance evolved and I immediately began antiviral treatment. Once again, I realized I should never forget to consider how common this virus can be.
References
- Xu F, Schillinger JA, Sternberg MR, et al. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J Infect Dis. 2002 Apr 15;185(8):1019-24.
- Straus SE. Overview: the biology of varicella-zoster virus infection. Ann Neurol. 1994;35 Suppl:S4-8.
- Yolton D, Haesaert S. Anti-Infective Drugs. In: Bartlett JD, Jannus SD (eds.). Clinical Ocular Pharmocology, 5th ed. Philadelphia: Butterworth-Heinemann; 2008:196-205.
- Wander AH, Centifanto YM, Kaufman HE. Strain specificity of clinical isolates of herpes simplex virus. Arch Ophthalmol. 1980 Aug;98(8):1458-61.
- Holmes EC. Viral evolution in the genomic age. PLoS Biol. 2007 Oct 2;5(10):e278..
- Yolton DP. Anti-Infective Drugs. In: Bartlett JD, Jannus SD (eds.). Clinical Ocular Pharmocology, 3rd ed. Philadelphia: Butterworth-Heinemann; 1995:281-9.
- Kollias CM. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol. 2014 Nov 12. [Epub ahead of print]
- Lee WB, Liesegang TJ. Herpes Zoster Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ (eds.). Cornea, 3rd ed. St. Louis: Mosby; 2011:985-1000.
- Kaufman HE, Rayfield MA. Viral conjunctivitis and keratitis: herpes simplex virus. In: Kaufman H (eds.). The Cornea. New York: Churchill Livingstone; 1988.
- Holland EJ, Schwartz GS, Neff KD. Herpes Simplex Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ (eds.). Cornea, 3rd ed. St. Louis: Mosby; 2011:953-84.
- Sneader W. Drug discovery: a History. New York: Wiley; 2005:258.
- Kaufman HE, Heidelberger C. Therapeutic antiviral action of 5-trifluoromethyl-2’-deoxyuridine. Science. 1964 Aug 7;145(3632):585-6.
- Elion GB. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12 Suppl B:9-17.
- Liesegang T. Diagnosis and therapy of herpes zoster ophthlamicus. Ophthalmology. 1991 Aug;98(8):1216-29.
- Colin J. Ganciclovir ophthalmic gel (Virgan; 0.15%) in the treatment of herpes simplex keratitis. Cornea. 1997 Apr;16(4):393-9.
- Kimberlin DW, Whitley RJ. Antiviral therapy of HSV-1 and -2. In: Arvin A, Campadelli-Fiume G, Mocarski E (eds.). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007:1153-74.
- Wagstaff AJ, Faulds D, Goa KL. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994 Jan;47(1):153-205.
- Soul-Lawton J, Seaber E, On N, et al. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995 Dec;39(12):2759-64.
- Obara Y. Distribution of herpes simplex virus types 1 and 2 genomes in human spinal ganglia studies by PCR and in situ hybridization. J Med Virol. 1997 Jun;52(2):136-42.
- Waggoner-Fountain LA, Grossman LB. Herpes simplex virus. Pediatr Rev. 2004 Mar;25(3):86-93.
- Liesang TJ, Melton J III, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Arch Ophthalmol. 1989;107:1155-9.
- Liesang TJ. Epidemiology of ocular herpes simplex. Arch Ophthalmol. 1989;107:1160-5.
- Barron BA. Herpetic eye disease study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994 Dec;101(12):1871-82.
- Liesang TJ. The varicella-zoster virus disease. Contemp Ophthalmol. 2006;5:1-7.
- Jumaan AO, Yu O, Jackson LA. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992-2002. J Infect Dis. 2005 Jun 15;191(12):2002-7.
- Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011 Feb 1;52(3):332-40.
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008 Feb;115(2 Suppl):S3-12.
- Zhou F, Harpaz R, Jumaan AO, et al. Impact of varicella vaccination on health care utilization. JAMA. 2005 Aug 17;294(7):797-802.
- Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008 Sep;122(3):e744-51.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013 Dec 1;208(11):1859-68.
- Edmunds WJ. The effect of vaccination on the epidemiology of varicella zoster virus. J Infect. 2002 May;44(4):211-9.
- Quirk M. Varicella vaccination reduces risk of herpes zoster. Lancet Infect Dis. 2002 Aug;2(8):454.
- Oxman MN. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005 Jun 2;352(22):2271-84.
- Duan R. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008 Sep 1;198(5):659-63.
- Kimberlin DW, Crumpacker CS, Straus SE, et al. Antiviral resistance in clinical practice. Antiviral Res. 1995 Apr;26(4):423-38.
- The Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for iridocyclitis caused by herpes simplex virus. Arch Ophthalmol. 1996 Sep;114(9):1065-1072.
- Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000 Aug;118(8):1030-6.
- Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998 May;339(5):300-6.
- Uchoa UB. Long-term acyclovir use to prevent recurrent ocular herpes simplex virus infection. Arch Ophthalmol. 2003 Dec;121(12):1702-4.
