 |
History
A 67-year-old African female presented with a chief complaint of sudden-onset blurred vision OS. Her history was positive for mild cataracts; otherwise, she had excellent ocular health. Her systemic history was positive for hypertension, for which she was adequately controlled with medication.
Diagnostic Data
Her best-corrected entering visual acuities were 20/20 OD and 20/400 OS at distance and near. Her external examination was normal with the exception of the facial confrontation field, which revealed a large relative central scotoma. There was no evidence of afferent pupillary defect. The biomicroscopic examination of the anterior segment was normal with mild, grade II lenticular opacities OU. Goldmann applanation tonometry measured 15mm Hg OU. The pertinent posterior pole pathology OS is demonstrated in the photograph.
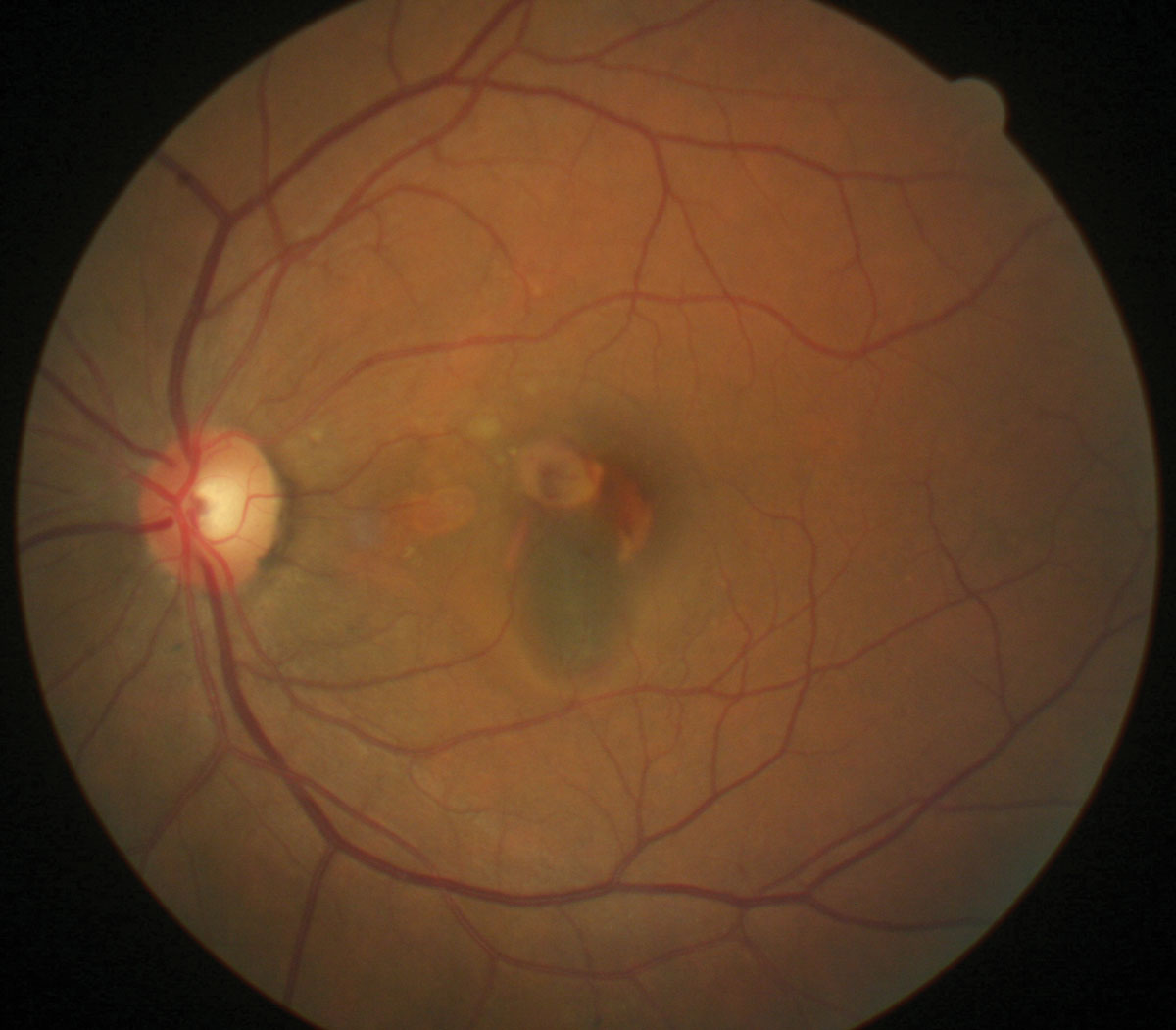 |
What’s causing the presentation shown here? What is the best course of action? Click image to enlarge. |
Findings
The diagnosis in this issue is idiopathic polypoidal choroidal vasculopathy (IPCV) or posterior uveal bleeding syndrome. IPCV is a typically unilateral disease that produces numerous periodic bouts of retinal pigment epithelium (RPE) detachment with or without hemorrhage secondary to serosanguinous leakage of the peripapillary region.1-13 A macular variant has been identified.5-7,13
The disease has also been associated with the risk factor of smoking and the ocular findings of myopic degeneration, the presence of staphyloma and a tilted disc.7,8,13 Though frequently mistaken for the processes of age-related macular degeneration (AMD), its differentiation is based on age of onset (i.e., affecting younger individuals) and absence of drusen, as well as its characteristic angiographic and OCT findings.1-6,13
Once believed to be a disease of middle-aged Black women, this presentation is now recognized to generically affect populations with increased pigmentation with some references recognizing a prevalence for the Asian population.1-13 The entity has a documented incidence of 8% to 13% in White patients with cases also reported in Irish, French, German and Italian nationalities.3
IPCV has no obvious gender predilection.1-6 The natural course of the disease often follows a remitting-relapsing course, which is clinically associated with chronic, multiple recurrent serosanguineous detachments of the RPE, associated neurosensory retinal detachment and occult subretinal neovascularization.4 In the absence of macular lesions, the long-term visual prognosis is remarkably good.1-12
Clinical Presentation
Funduscopically, IPCV presents as subretinal orange nodules (polyps) within the choroidal vasculature.1-13 Angiographically, intravenous FA poorly delineates the choroidal circulation. The technique of indocyanine green angiography (ICGA) introduces a dye capable of permeating the RPE and overlying fluids and because of its ability to assume a molecular size by binding to albumin. In this form, the dye cannot escape the choriocapillaris, allowing detailed imaging of these structures.12-18 Active IPCV will appear in the ICGA as a hot spot at the location of the leaking polyps.12-18 Since IPCV sometimes progresses to frank occult choroidal neovascularization, ICGA remains a powerful and useful tool.12-18
Polypoidal choroidal vasculopathy has been considered by many as a distinct choroidal abnormality characterized by an intrachoroidal vascular network of vessels ending in polyp-like structures.1-18 Researchers have been able to discern two different patterns of vascularization.2,19 The first demonstrates feeder and draining vessels along with network vessels creating the characteristic findings seen in choroidal neovascularization (CNV).2,9 Points of focal dilatation on marginal vessels were comprised of polypoidal lesions.2 The second pattern demonstrated neither feeder nor draining vessels in the setting of far fewer network vessels.2 Again, the points of deformation of the network vessels appeared as polypoidal lesions.2
The researchers believe the first pattern represents a variant of deformed CNV.2,9 The second pattern is more unique and is postulated to result from abnormalities of the choroidal vessels, such as abnormal dilatation and hyalinization of the vessels in the setting of exudative changes in the blood plasma.2 Further, changes in the basement membrane, showing deposits and granulomatous tissue, suggest that at least in some cases the condition arises from hyalinized arteriolosclerosis of choroidal vessels.2,10,11 The suspected pathophysiology in cases of staphyloma and tilted disc is induced blood-flow disturbance to the region.6
Once the abnormal vessels form, it is thought they invade Bruch’s membrane to form polypoidal network vessels that push the RPE upward secondary to the increase in intravascular pressure created by the dilated vessels and via exudation from the vessels in the choroidal network.2,19
Causes
The etiology of subretinal disease may be infectious (e.g., toxoplasmosis, histoplasmosis), inflammatory (e.g., acute multifocal placoid retinal pigment epithelium chroioretinopathy), viral (e.g., multiple evanescent white dot syndrome), parasitic (e.g., nematode, Toxocara canis), dystrophic (e.g., geographic atrophy, AMD), traumatic (e.g., choroidal rupture) or idiopathic.
The key to treatment is identifying the underlying cause. This is accomplished by combining the history with examination data and observable characteristic clinical signs. In the event fluid and hemorrhage obscure choroidal details, OCT, FA and ICGA can assist in diagnosis as well as guide treatment and improve post-treatment-monitoring.12,20-25
When epidemiology and clinical appearance suggest IPCV, the test of first choice is ICGA.24,25 It can be done as a stand-alone or in combination with FA. ICGA was developed more than 30 years ago, but has been used commonly for less than 10 years.24,25 The tricarbocyanine dye is injected intravenously and is imaged as it passes through ocular vessels. An excitation filter permits the capture of its pooling or leakage.12,25-27 The large molecular characteristics of the dye itself, its ability to bind to albumin and remain inside the large choroidal vessels, and its fluorescent properties all enable viewing in the presence of bleeding.12,25-27
Neovascularization is observed using ICGA as either focal hyperfluorescence (hot spot) or diffuse hyperfluorescence (plaque). Scanning laser ophthalmoscopy imaging allows for better visualization of feeder vessels, allowing even more selective treatment.19
Treatment
Photodynamic therapy (PDT) using the angio-occlusive benzoporphyrine derivative verteporfin, by itself or in combination with anti-VEGF injection, remains the standard of care treatment.24,28-33 While the addition of the anti-VEGF agent has not shown a significant ability to improve outcomes or reduce polypoidal lesion recurrence, the EVEREST and PEARL studies (along with others) demonstrated the agent’s suitability for limiting PDT-related subretinal hemorrhages.29-33 For this reason, co-therapy seems to be gaining acceptance over PDT alone.29-33
Continuous monthly anti-VEGF treatment has also been studied.34 While the treatment is well tolerated and yields reduced polypoidal lesions, visual stabilization, resolution of subretinal hemorrhage and reduced macular edema, branching choroidal vessels may persist.34
Laser photocoagulation targeted to the feeder vessels supplying IPCV lesions and extrafoveal IPCV lesions is also recognized as an effective treatment.6,35 It is also used as a last resort when the PDT/anti-VEGF strategy fails.35
A few pointers:
- Since choroidal neovascular membranes are capable of growing at a rate of 10µm to 15 µm per day, prompt referral to a retina specialist is paramount upon suspicion of any CNV with the ability to breach the foveal integrity.
- All modern imaging techniques (ICGA, FA, OCT) have a potential role in the accurate diagnosis and following of IPCV.
- Patients found to have exudative, hemorrhagic retinopathy, without signs of active inflammation or precursors to age-related pathology, should be considered suspicious for IPCV.
This patient was treated with the standard course of anti-VEGF plus PDT treatment, resulting in a mild restoration of visual acuity.
| 1. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina. 2012;32 Suppl 1:1-8. 2. Yuzawa M. Polypoidal choroidal vasculopathy. Nihon Ganka Gakkai Zasshi. 2012;116(3):200-31. 3. Ciardella AP, Donsoff IM, Huang SJ, et al. Polypoidal choroidal vasculopathy. Surv Ophthalmol. 2004;49(1):25-37. 4. McCleary CD, Guier CP, Dunbar MT. Polypoidal choroidal vasculopathy. Optometry. 2004;75(12):756-70. 5. Escaño MF, Fujii S, Ishibashi K, et al. Indocyanine green videoangiography in macular variant of idiopathic polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2000;44(3):313-6. 6. Stangos AN, Gandhi JS, Nair-Sahni J, et al. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010;150(5):666-73. 7. Moorthy RS, Lyon AT, Rabb MF, et al. Idiopathic polypoidal choroidal vasculopathy of the macula. Ophthalmology. 1998;105(8):1380-5. 8. Mauget-Faÿsse M, Cornut PL, Quaranta El-Maftouhi M, Leys A. Polypoidal choroidal vasculopathy in tilted disk syndrome and high myopia with staphyloma. Am J Ophthalmol. 2006;142(6):970-5. 9. Quaranta M, Mauget-Faysse M, Coscas G. Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol 2002;134(2):277-80. 10. Costa RA, Navajas EV, Farah ME, et al. Polypoidal choroidal vasculopathy: Angiographic characterization of the network vascular elements and a new treatment paradigm. Prog Retin Eye Res 2005;24(5):560-86. 11. Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol 2005;89(5):602-7. 12. Stanga PE, Lim JL, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmol. 2003;110(1):15-21. 13. Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29(1):19-29. 14. Kumar A, Nainiwal S, Prakash G. Indocyanine green angiography in age-related macular degeneration. Bombay Hosp J. 2002;44(3):333-9. 15. Alexander LJ. Exudative and nonexudative macular disorders. In: Alexander LJ, ed. Primary care of the posterior segment, 3rd Ed. New York, McGraw-Hill 2002:75-208. 16. Fernandes LH, Freund KB, Yannuzzi LA, et al. The nature of focal areas of hyperfluorescence or hot spots imaged with indocyanine green angiography. Retina 2002;22(5):557-68. 17. Berger JW, Maguire MC, Fine SL. The macular photocoagulation study. In: Kertes, P J, Conway MD, eds. Clinical trials in ophthalmology: a summary and practice guide, 1st Ed. Philadelphia; Lippincott, Williams and Wilkins 1998:71-96. 18. Nishijima K, Takahashi M, Akita J, et al. Laser photocoagulation of indocyanine green angiographically identified feeder vessels to idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 2004;137(4):770-3. 19. Okubo A, Hirakawa M, Ito M, et al. Clinical features of early and late stage polypoidal choroidal vasculopathy characterized by lesion size and disease duration. Graefes Arch Clin Exp Ophthalmol. 2008;246(4):491-9. 20. Conrath J, Ridings B. Indocyanine green angiography in age-related macular degeneration. J Fr Ophtalmol. 2003;26(8):854-63. 21. Ueno C, Gomi F, Sawa M, Nishida K. Correlation of indocyanine green angiography and optical coherence tomography findings after intravitreal ranibizumab for polypoidal choroidal vasculopathy. Retina. 2012;32(10):2006-13. 22. Chae JB, Lee JY, Yang SJ, et al. Time-lag between subretinal fluid and pigment epithelial detachment reduction after polypoidal choroidal vasculopathy treatment. Korean J Ophthalmol. 2011;25(2):98-104. 23. Coppens G, Spielberg L, Leys A. Polypoidal choroidal vasculopathy, diagnosis and management. Bull Soc Belge Ophtalmol. 2011;(317):39-44. 24. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008;19(3):208-12. 25. Windisch R, Windisch BK, Cruess AF. Use of fluorescein and indocyanine green angiography in polypoidal choroidal vasculopathy patients following photodynamic therapy. Can J Ophthalmol. 2008;43(6):678-82. 26. Hutchinson, J.K., Street, M., Gurwood, A.S., Myers, M.D. Face off: Fluorescein vs. OCT. Review of Optometry 2011; 148(9): 62-75. 27. Slakter JS, Yannuzzi LA, Guyer DR. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995;6(3):25-32. 28. Ziemssen F, Heimann H. Evaluation of verteporfin pharmakokinetics--redefining the need of photosensitizers in ophthalmology. Expert Opin Drug Metab Toxicol. 2012;8(8):1023-41. 29. Koh A, Lee WK, Chen LJ, et al. EVEREST Study: efficacy and safety of verteporfin photodynamic therapy with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012;32(8):1453–1464 30. Gomi F, Sawa M, Wakabayashi T, et al. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150(1):48-54. 31. Kim SJ, Yu HG. Efficacy of combined photodynamic therapy and intravitreal bevacizumab injection versus photodynamic therapy alone in polypoidal choroidal vasculopathy. Retina. 2011;31(9):1827-34. 32. Lee YH, Lee EK, Shin KS, et al. Intravitreal ranibizumab combined with verteporfin photodynamic therapy for treating polypoidal choroidal vasculopathy. Retina. 2011;31(7):1287-93. 33. Tomita K, Tsujikawa A, Yamashiro K, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol. 2012;153(1):68-80. 34. Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94(3):297-301. 35. Gemmy Cheung CM, Yeo I,Li X, et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155(2):295-304. |

