 |
|
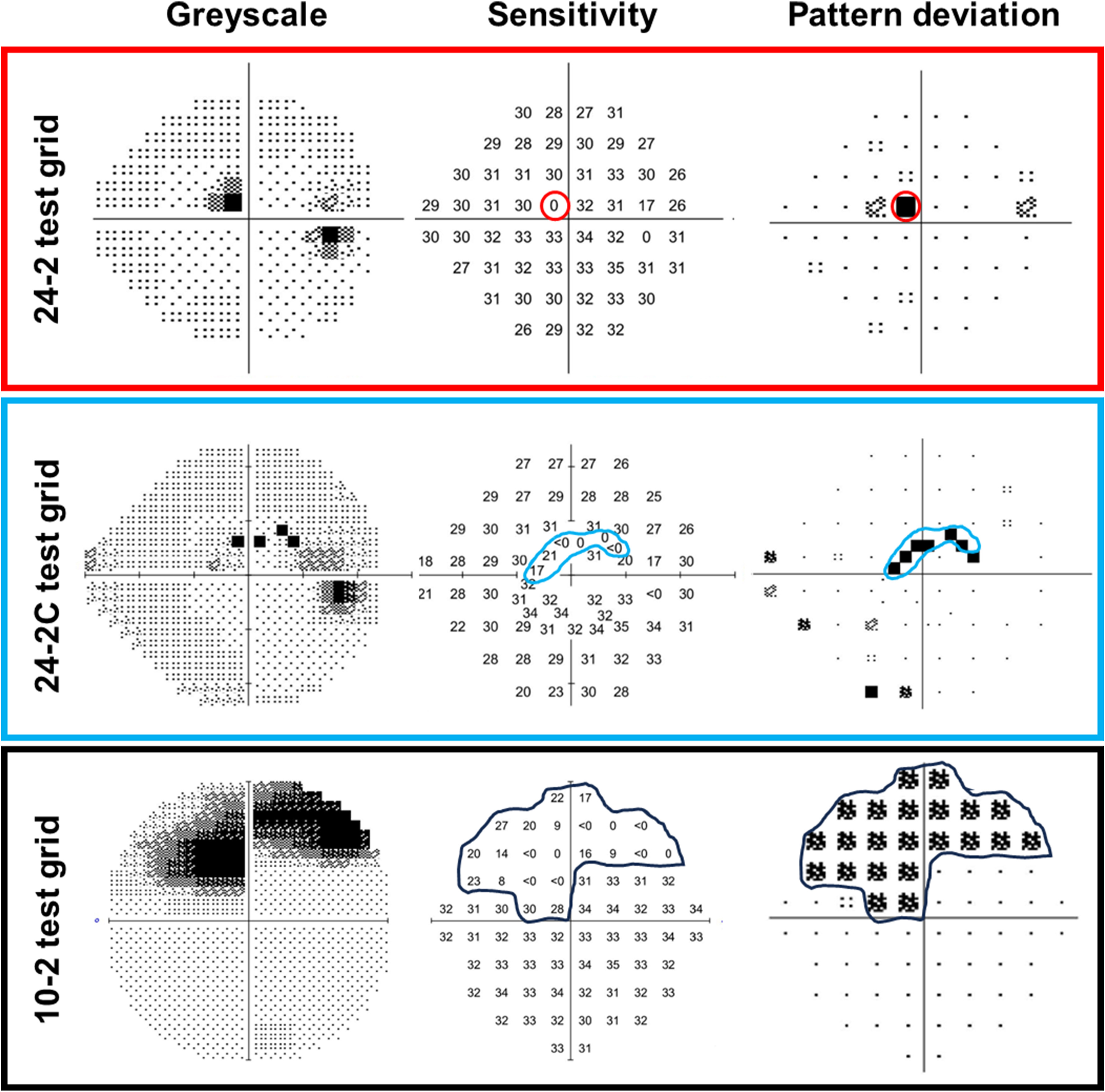
One of the many points illustrated in the paper concerns the influence of stimulus size and test grid selections. These images from the paper give examples comparing glaucomatous visual field defects across different test grids for a 34-year-old patient with low-tension glaucoma and a central visual field defect when using three static perimetry test grids: the 24-2, 24-2C and 10-2. Photo: Jack Phu, OD, PhD/Prog Retin Eye Res. October 15, 2024. Click image to enlarge. |
Standard automated perimetry (SAP) remains essential in the functional assessment of visual field changes brought on by diseases of the visual pathway, most notably glaucoma. A recent review, published online ahead of print in Progress in Retinal and Eye Research, comprehensively describes the collective knowledge amassed on perimetry to date. Over the course of roughly 140 manuscript pages, a team of experts from the School of Optometry and Vision Science at the University of New South Wales in Australia systematically dissect perimetry as both a psychophysical experience and a clinical tool.
“Although we focused on the vision science aspects of perimetry, we wanted to link this back to clinical practice so clinicians would have a greater appreciation of where we are headed,” explains Jack Phu, OD, PhD, lead author of the paper.
Enduring issues for researchers and clinicians to address, the paper notes, include the optimization of SAP parameters for maximizing defect detection, the influence of subjective behavior on the response, structure-function discordance and age- and disease-related changes of the visual pathway. Limitations include its relatively insensitivity to early visual field loss, which could confound accurate clinical diagnosis of glaucoma, as well as variability in sensitivity measurements and long test duration. The paper proposes that optimized structure-function models could provide another avenue in the future for personalizing the testing approach for individual patients.
“Currently, SAP in clinical practice is predominated by projection-type perimeters, such as using the Humphrey Field Analyzer or the Octopus Perimeter,” says Dr. Phu. “Although widely used, we know that there are significant limitations to the techniques in terms of their ability to represent a person’s visual function.”
Dr. Phu notes that the parameters used in SAP are relatively uniform across many instruments—such as using a white, circular, hard-edged stimulus of fixed size (Goldmann size III) and presentation duration (200ms)—but these represent a historical precedent set in the 1980s that has remained unchanged despite evidence that tailoring the parameters could improve our ability to detect sensitivity loss. “With increasing test eccentricity, it would be appropriate to scale up the stimulus sizes,” notes Dr. Phu. “Newer perimetry methods are acknowledging this limitation.”
The paper highlighted that alternative testing platforms, such as portable tablet or headset monitors, employ newer technology with customizable and flexible testing protocols that are not necessarily shackled to precedent set in SAP, including choices made decades ago regarding stimulus parameters, test grids and thresholding algorithms.
Ongoing research is investigating ways to customize optimal patient-specific stimuli for perimetry. “As more evidence in this area develops, the combination of testing platforms other than traditional projection perimeters with customizable stimulus parameters may lead to a paradigm shift in more exquisite visual field testing, changing the definition of ‘standard’ in standard automated perimetry,” the study authors wrote in their paper.
Dr. Phu explains that some of the reviewed studies also suggested that background luminance levels lower than SAP, such as in the mesopic or scotopic range, may be better for identifying visual field defects in the context of pathology. “These parameters could also be important for linking results of these clinical tests under low light conditions with everyday visual functioning, such as seeing in dim environments,” he says.
The team’s review paper also presented empirical evidence for test grid selection in patients who may benefit from central visual field testing using the 10-2 and suggested that customized grids could become the norm for patients. “The appropriateness of the 24-2 and 10-2 test grids—the de facto standards for glaucoma—is a topic of ongoing investigation,” explains Dr. Phu. “Both have their strengths and limitations, and using them represents a trade-off in time in clinical practice.”
Studies that examined different test grid densities imply that there are both “high-yield and futile visual field test locations,” the researchers emphasized in their paper. “There is also likely a dose-dependent relationship, such that more test locations are more likely to identify defects at the cost of requiring time for testing.”
Although newer thresholding paradigms could potentially reduce this test duration, they speculated that a potential role for this approach is to target specific regions of interest, such as following guidance by structural defects. The team noted that the question of structure-function relationships in this context relates to understanding the balance between the number of test locations and practical test durations.
“Optimizing the structure-function relationship is important in increasing clinician confidence in clinical diagnosis, such as in the identification of glaucoma,” Dr. Phu remarks. “In the future, optimized structure-function models could provide another avenue for personalizing the testing approach for individual patients.”
Dr. Phu and colleagues believe that the future of perimetry will be an increasingly personalized experience for the patient. “Eventually, we will be able to customize a perimetry test that is tailored to the patient’s ocular structures, pathology and longitudinal progression to enhance clinical care,” he says.
| Click here for journal source. |
Phu J, Khuu SK, Nivison-Smith L, Kalloniatis M. Standard automated perimetry for glaucoma and diseases of the retina and visual pathways: current and future perspectives. Prog Retin Eye Res. October 15, 2024. [Epub ahead of print]. |

