Cataract surgery has become one of the most frequently performed and most successful surgical procedures, with overwhelmingly positive outcomes.1 While the complication rate after cataract surgery is relatively low, the sheer number of procedures coupled with a large group of comanaging optometrists merits a review of potential postoperative complications.1 Most postoperative problems may be adequately treated by the comanaging OD, though some may require referral back to the surgeon. Postoperative complications are best characterized by the timeframe when they occur and by frequency.
Early Common Complications
Though the official postoperative period begins immediately after surgery concludes, we will consider ‘early’ complications as roughly spanning the first four weeks. Generally the first postoperative check occurs within 24 hours of surgery. At that appointment, vision will often be described as qualitatively blurry. During this early period, topical steroids and antibiotics are routinely started four times a day, with or without a non-steroidal anti-inflammatory drug (NSAID)—dosed as the examining doctor deems necessary. The frequency and timing of subsequent postoperative checks depends on how the patient heals, essentially on these aspects:
Case 1. Retained Nuclear Fragment | |
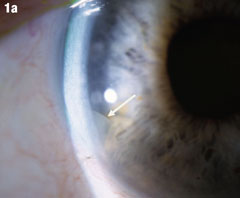 | Although this 77-year-old male’s uncomplicated bilateral cataract surgery was performed in 2007, the fragment in his right eye wasn’t seen until almost two years later. A nuclear fragment in the anterior chamber (Figure 1a) after cataract surgery is a rare, but well-reported event. Figure 1b shows the eye after it was removed. Iritis may occur years after surgery. If it occurs in a patient who has had phacoemulsification, that should prompt a careful examination for nucleus fragments in the anterior chamber. Corneal edema may be associated, but was not in this case. Click images to enlarge. |
 | |
Cell/flare. After surgery, at least a small amount of cell and flare will always be present in the anterior chamber. This decreases the clarity of the initial refractive outcome. This is not truly considered a complication of cataract surgery, but rather an expected result. Its sequelae often include complaints of blurry vision or light sensitivity. Topical steroids are prescribed and tailored for this.
Corneal edema. Virtually all patients will present with some level of corneal edema. This is commonly localized around the corneal incisions, but certainly can present diffusely anywhere on the cornea. This can range from relatively superficial epithelial swelling to full-thickness edema with Descemet’s folds, especially when the surgery is longer in duration or the cataract is more dense. While most postoperative corneal edema will resolve over time, most physicians prefer to treat corneal edema with topical steroids, customizing the dosage to the level of corneal edema present. This can be done by either raising the frequency of drop administration or by changing to a more potent steroid (i.e., prednisolone to difluprednate). Raised intraocular pressure (IOP) can also manifest as diffuse microcystic epithelial edema.2 One reason an NSAID is often given is for discomfort related to the incisions or corneal damage.
Elevated IOP. Elevated intraocular pressure is not an uncommon problem following cataract surgery. It is the most frequent postoperative complication that demands treatment.3 Statistics vary, but as many as 18% to 45% of patients experience a pressure greater than 28mm Hg initially, but one that often returns to their baseline by 24 hours postoperatively either with or without treatment.3 When IOP approaches 40mm Hg, sometimes the paracentesis wound may need to be ‘burped’, which necessitates some previous training or a trip back to the surgeon. When the corneal edema coincides with some level of raised IOP, a delicate balance of ocular hypotensive and steroid may be required. For example, a highly edematous, 3+ to 4+ fold cornea with an IOP of 35mm Hg may demand sustained prednisolone dosage with an added beta blocker or carbonic anhydrase inhibitor, especially if the nerve is suspicious for glaucoma. Frequent pressure and cornea checks may be needed to adjust the drops to the proper dosage. Causes for elevated IOP within 24 hours of surgery generally include retained viscoelastic or pre-existing glaucoma problems.
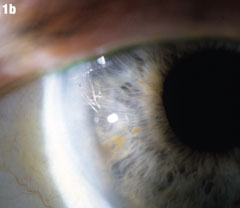
Case 2. Positioning Problems | |
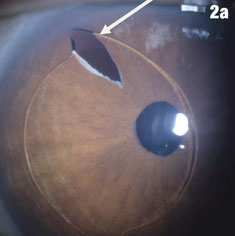 | 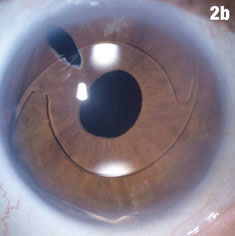 |
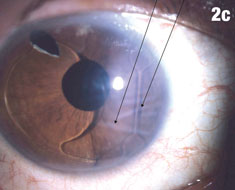 | 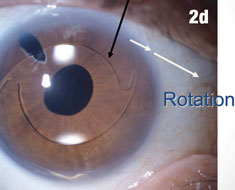 |
| Figure 2a shows a patient with an IOL out of position and in the peripheral iridectomy. Figure 2b shows the same eye after the IOL was restored to its proper position. Figure 2c shows how this IOL, out of position, caused corneal folds. The arrows in Figure 2d show how the lens was rotated out of the iridectomy. Click images to enlarge. | |
It may be challenging to determine the correct balance in the treatment drop regimen in patients who present with high IOP due to steroid response, though this condition is usually elucidated at the one-week follow up after the steroid has been used for some time, not the 24-hour check-up.4 Those patients with steroid response, assuming normalization of the cornea and anterior chamber, may need to taper the steroid quicker or be switched to a less potent steroid (e.g., from difluprednate 0.05% to prednisolone acetate 1% or loteprednol etabonate 0.5%), or with the addition of a topical hypotensive, such as a beta blocker or carbonic anhydrase inhibitor or alpha-2 agonist. If the IOP is greater than 30mm Hg, another 24-hour pressure check may be required. A modified paracentesis procedure or a burping of the wound should be considered if the initial postoperative IOP is 40mm HG or greater. If not high, the next check may take place a few days later—at which point the steroid and hypotensive regimen can be revised.5
Early Rare Complications
Iris prolapse. A truly rare early postoperative complication, iris prolapse most commonly from inadequate wound closure, accidental trauma or raised IOP.4 If the iris tissue is seen less than 48 hours post surgery, its tissue can be repositioned by the cataract surgeon.4 If the iris prolapse is of longer duration, the prolapsed section may need to be excised, a procedure left for the operating room.6
Case 3. Retained Cortical Fragment | |
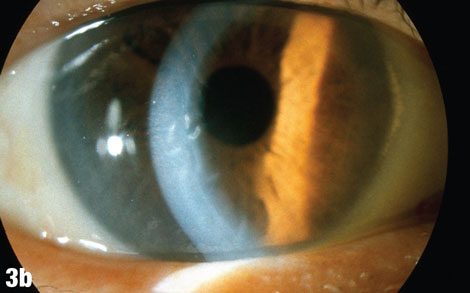
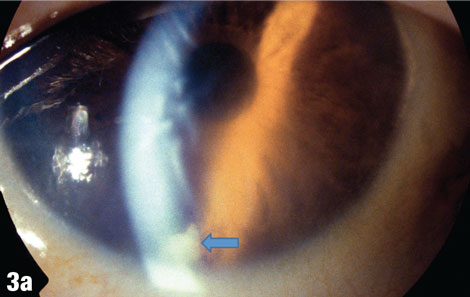 | This 75-year-old male patient presented one week after having a cataract removed from his right eye, with a complaint of blurry vision in that eye only. The blue arrow in Figure 3a shows a cortical fragment in the anterior segment. After he returned to the surgeon to have the fragment removed, his vision cleared up. Figure 3b shows the patient’s right eye following the removal. |
 | |
Wound leaks. These are also rare, and can present in a number of different ways. Signs include poor vision, IOP less than 8mm Hg, complaints of epiphora and shallow anterior chamber. The easiest way to clinically identify a wound leak is with the instillation of fluorescein dye. Close inspection of the fluorescein-surrounded wound under cobalt blue filter light will show a dark band of negatively dyed fluid, or aqueous, running from the wound. Management of the leak depends on the severity, cause and timing of the leak—usually small wound leaks resolve within 24 hours to 48 hours, with the only intervention being decreasing the steroid dosage or strength.5 Moderate leaks may require the use of a bandage contact lens, or an added cycloplegic or aqueous inhibitor to tamponade the flow. In either mild or moderate case, diligent follow up every 24 hours is recommended. If the leak is significant and the chamber appears shallow with a low IOP, the patient should be sent back to the surgeon for repair.7
Toxic anterior segment syndrome (TASS). Thankfully, this is a very rare early occurrence usually appearing 12 hours to 72 hours after cataract surgery.2 Anterior segment inflammation in this case is quite severe, often with hypopyon, fibrinous uveitis and significant corneal swelling due to endothelial cell damage.2 It is sterile, minimally painful and, as the name implies, does not present with vitreous involvement—it is localized to the anterior segment. When it does occur, it’s almost always after uneventful cataract or anterior segment surgery.8 The culprits can involve contaminated surgical equipment, inadequately sterile drops or solutions introduced into the eye during the procedure, or other foreign substances such as talc from surgical gloves.9
Management of TASS varies in the literature but generally requires high doses of topical steroids every hour; some studies advocate frequent NSAIDs or even oral steroids.2,10 It is vital to differentiate TASS from infectious endophthalmitis, also rare, and whose incidence is from 0.05% to 0.4% in different studies.2 From an anterior perspective, the two appear fairly similar.
Case 4. Pseudoexfoliation |
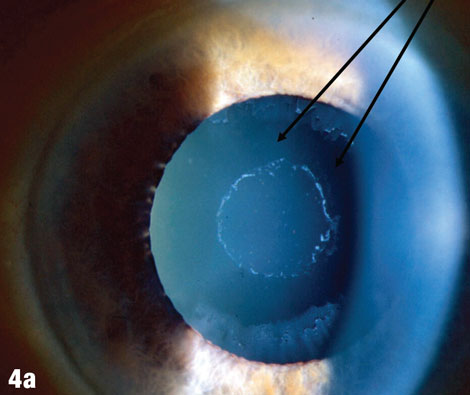 |
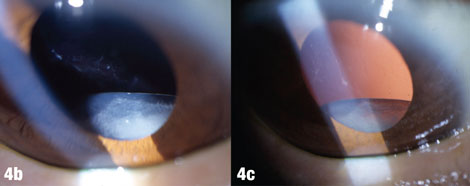
| The arrows in Figure 4a shows where deposits have been rubbed off the lens by the iris. Figures 4b and 4c show a case where pseudoexfoliation caused complete IOL dislocation seven years after cataract surgery. |
 |
Early acute endophthalmitis. Though TASS appears roughly 24 to 72 hours after surgery, endophthalmitis occurs three to seven days post surgery.2 Both will present with blurred vision, corneal edema and anterior chamber reaction and likely hypopyon. In endophthalmitis, the corneal edema will be greater, accompanied by larger hypopyon—though depending on the duration and severity, the anterior segment findings may be confounding.2 The main difference is the presentation of the vitreous, where endophthalmitis will present with some level of vitritis, but TASS will have a clear vitreous. In terms of treatment, unlike TASS, endophthalmitis will not respond to steroids and will require referral to the surgeon or retinal specialist for either intravitreal broad spectrum antibiotics with likely vitreous sample taken, or pars plana vitrectomy. Systemic or periocular antibiotics may be used as well.2,11
Retained lens fragments. Retained lens fragments can appear any time in the postoperative period, from day one through months to even years later. They can present anywhere in the eye—anteriorly if the capsule is intact, posteriorly if the capsule has ruptured. The fragments are not always easy to see, as they may be very small and may hide within the angle or behind the iris and cause symptoms such as blurred vision, photophobia, tearing and redness.
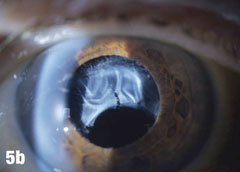
Case 5. IOL Dislocation and Corneal Trauma | |
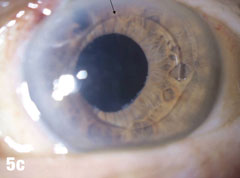
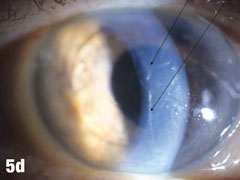
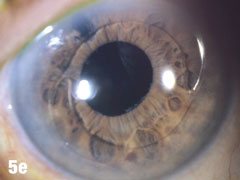
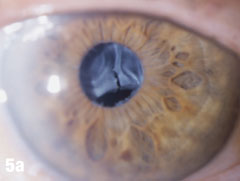 | This 84-year-old female patient underwent cataract extraction four years prior and a YAG capsulotomy one and a half years ago in her left eye. She had a visual acuity of 20/30 OS, but reported seeing a “film” over her eye (Figure 5a). The dilated view (Figure 5b) reveals the problem: her superior zonules ruptured and her posterior chamber IOL and capsular bag came loose. You can better see the opaque anterior capsule in this view. The patient had two surgical options. She could have the dislocated posterior chamber IOL sewn back into to place, risking erosion of the sutures, or she could have an anterior chamber IOL installed. The second option would put her at risk for glaucoma and iritis, but has a shorter recovery time. She was fitted for an anterior chamber IOL. Figure 5c shows her dilated eye two weeks after the IOL was placed. Unfortunately, she also developed endothelial striae as a result of surgical trauma (Figure 5d), which was later resolved after a short course of steroid drops (Figures 5e). |
 | |
 | |
 |  |
| Click images to enlarge. | |
If the fragment is in the anterior chamber, the patient may present with symptoms of blur with a sectorally edematous cornea and an anterior chamber reaction. If possible, gonioscopy should be completed to visualize the angle and trabecular meshwork in search of the fragment.
If the retained material is cortical, it may appear light yellow to white in color and translucent. If the fragment is nuclear, it may appear darker yellow/brown and more opaque. Small amounts of cortical material often can be followed closely with topical anti-inflammatory drops without surgical intervention.12 When the fragment is nuclear, anterior to the lens and there is a considerable amount of inflammation, the particle should be removed via surgical intervention by the original operating ophthalmologist.
A large posterior fragment may need removal with pars plana vitrectomy. If the patient remains to be followed by the OD, they should be followed closely for the development of cystoid macular edema (CME), retinal detachment (RD), ocular hypertension or corneal decompensation.12
Late Common Complications
Posterior capsular opacification (PCO). Sometimes associated with Elschnig’s pearls, this is the most frequent late complication from cataract surgery.13 Incidence of PCO varies in the literature from 14% to 60%, and may be more frequent in younger patients, myopes, those with diabetes, those with previous ocular surgeries and those with greater amounts of posterior subcapsular or cortical cataracts.13
The severity of PCO is evaluated in many ways using simple observation under the slit lamp light, either under direct slit beam or via retroillumination. The most important grading scale is subjective—determining if the patient’s best corrected vision has become problematic. One grading system uses a 0 to 3 scale, where 0=absent, 3=dense white.14 A more commonly used method is a four point scale, where 1=no or slight PCO without reduced red reflex and 4=severe fibrosis covering the visual axis.14
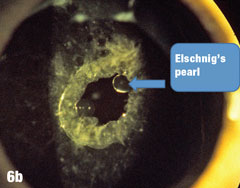
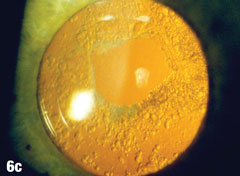
Case 6. Elschnig’s Pearls After YAG Capsulotomy | |
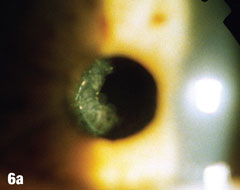 | Epithelial cells may continue to grow on the posterior capsule, forming what are known as Elschnig’s pearls. The epithelium secretes a clear material that gets trapped within the epithelial sheets. In the case presented here, after the first YAG procedure, cells grew into the visual axis and decreased acuity in the right eye. Figure 6a shows the undilated initial presentation of a 69-year-old female patient’s right eye one year after the YAG capsulotomy. The dilated view in Figure 6b clearly shows Elschnig’s pearls, for which the patient underwent repeat capsulotomy. Figure 6c offers a retroview, exposing multiple pearls that remain in the posterior opacification capsule. Note how the second YAG procedure created an enlarged clear central area without pearls. The patient’s vision was restored to 20/20. |
 | |
 | |
The mechanism of PCO development is usually secondary to a proliferation and migration, or growth of residual lens epithelial cells left on the anterior capsule after surgery.13 Though these residual cells themselves are not dangerous to the implant or the eye, they can scatter light and decrease the patient’s best corrected vision. They often cause glare and hamper the OD’s view of the fundus.14 Treatment for PCO, should it become visually significant, is Nd:YAG laser posterior capsulotomy, commonly referred to as a YAG. Though the laser procedure is quick and painless for the patient, it is not without risk. Retinal complication concerns such as lattice, holes and weak spots should be addressed prior to proceeding with laser due to risk of retinal detachment.15
Cystoid macular edema. Pseudophakic CME, or Irvine-Gass syndrome, is less common in recent years and it can vary from 1% to 30%, and generally peaking at six weeks postoperatively.16-18 Patients may present with best corrected vision that has worsened from previous visits. Dilated exam and OCT can confirm parafoveal cystic spaces.18 On fluorescein angiogram, the macula may show a characteristic petalloid pattern of leakage.16 Factors that are predictive in its development are previously compromised maculae—via diabetes, epiretinal membrane, other vascular event or preoperative use of prostaglandin analogs—as well as ruptured posterior capsule.18
As CME is thought to be an inflammatory process, the most common treatment is a regimen of topical nonsteroidals with or without the addition of topical steroids.18 The mean resolution of patients treated topically with an NSAID is approximately two months, though it can take longer.18 Rarely, CME requires intravitreal injection with corticosteroids or pars plana vitrectomy.18
Late Rare Complications
IOL dislocations. With improvements in IOL design and surgical technique, the incidence of subluxation has become more rare, estimated from 0.2% to 3%.19,20 Previously, IOL dislocations happened more often in the early postoperative period, but with the advent of intraoperative adjunctive devices such as capsular tension rings and iris hooks, progressive zonular dehiscence, and late ‘in-the-bag’ dislocation incidents have been increasingly reported, its risk increasing with the presence of pseudoexfoliation.19,20 The patient may or may not be symptomatic to the dislocation—most often with a sudden, painless loss of some level of vision.
If the complaint of blur is mild, and the lens is not posing a threat to the eye, many patients will use refractive means to treat—via contact lens or spectacle prescription. Surgical treatment for IOL dislocation, if intervention is warranted and wanted by the patient, is multifaceted. Depending on the type of lens originally implanted, sometimes it can be intraoperatively sutured to the iris or sclera. If the lens must be explanted, a sulcus, anterior chamber IOL, or sutured IOL may be implanted.19,20
Case 7. Retinal Detachment |
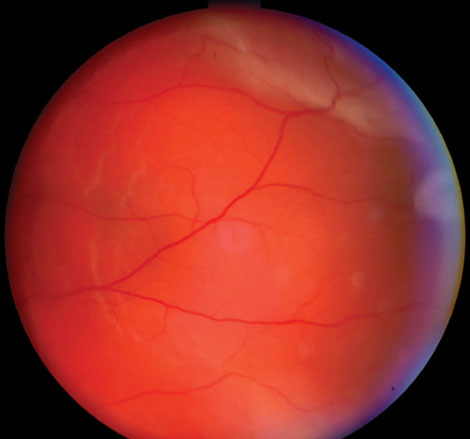 |
| This 57-year-old male’s retinal detachment occurred seven months after cataract surgery in his left eye. Detachment issues are uncommon in general, but are more common in patients younger than 60 years who have not yet had a posterior vitreous detachment or in patients whose axial length is 26mm or longer. Trauma is also associated with retinal detachment. This patient underwent a scleral buckle procedure and achieved a visual acuity of 20/20. Click image to enlarge. |
Retinal detachments, most commonly in younger patients without a posterior vitreous detachment, are an important late comorbidity associated with dislocated IOLs. Up to 6% of these patients will present with a concurrent RD, though many of those involved trauma.21
Capsular contraction syndrome. Anterior capsular phimosis (ACP), also known as capsular contraction syndrome or anterior capsule contraction syndrome, is quite uncommon with only a few cases reported in the literature. One study of 801 eyes had an incidence of 0.004%.22 ACP is due to remaining lens epithelial cells that lead to fibrous metaplasia and contraction, causing a reduction of the capsular opening.23 Vision can be impaired not only because of the fibrous nature of the opacification, but also due to the potential tilting, decentration or folding forces placed on the lens by the contracting capsule.23 The treatment for ACP, should the lens warpage merit intervention, is YAG laser ‘relaxing incisions’ at the edge of the capsulorhexis.22
Chronic corneal edema. Pseudophakic bullous keratopathy (PBK), or the development of irreversible corneal edema following cataract surgery, has decreased dramatically since the 1980s.24 The current incidence, as reported by the FDA, is 0.1%, though it was once as high as 1.5%.24 The risk of its occurrence depends on a multitude of factors; the endothelial cell integrity and presence of Fuchs’ greatly increases the chance. Lenses other than a posterior chamber IOL, such as iris-fixated and anterior chamber IOLs, also raise the risk.24,25
The prime feature of PBK is persistent stromal corneal edema with associated loss of endothelial cells, which progresses towards the intercellular epithelial region and forms characteristic bullae.24 Its treatment includes topical hyperosmotic agents, 2% and 5% sodium chloride solution and ointment, dosed based on the level of corneal edema.24 Sometimes, topical hypotensives such as beta blockers and alpha agonists decrease corneal edema; however, clinicians should avoid carbonic anhydrase inhibitors for the risk of increased endothelial toxicity, and both prostaglandin analogs and miotics can worsen the ocular inflammation.24 In cases of painful epithelial bullae, bandage contact lenses may be used. In terms of surgical care, penetrating keratoplasties were once the treatment of choice, but the newer lamellar endothelial keratoplasties (such as DSAEK and DMEK) have gained popularity and are now considered first line treatments.
Attentive postoperative cataract care is vital for the patient’s success, both short and long term. Crucial for this care is having a firm understanding of expected and unexpected complications, and their subsequent management. Be sure to contact the cataract surgeon for any sight-threatening medical complications or if there is any doubt as to treatment regimen.
Dr. Fabrykowski is a staff optometrist with Manhattan Eye, Ear and Throat Hospital Faculty Ophthalmology Practice, under Lenox Hill Hospital in New York City
Dr. Garston is a professor at the New England College of Optometry and an optometrist at MIT Medical Department. He is also a founding fellow of the Optometric Retina Society.
|
1. Erie JC. Rising cataract surgery rates: demand and supply. Ophthalmology. 2014;121(1):2-4. 2. Kurt E, Mayali H. Early post-operative complications in cataract surgery. In: Zaidi F, ed. Cataract Surgery. Rijeka: InTech;2013:245–58. 3. Hildebrand G, Wickremasinghe S, Tranos P, et al. Efficacy of anterior chamber in controlling early intraocular pressure spikes after uneventful phacoemulsification. J Cataract and Refract Surg. 2003;29:1087-92. 4. Kersey J, Broadway D. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond). 2006 Apr;20(4):407-16. 5. Ellis B, Lighthizer N. Learn to burp corneal wounds without a hiccup. Review of Optometry. 2016;153(2):84-9. 6. Byrd S, Singh K. Medical control of intraocular pressure after cataract surgery. J Cataract Refract Surg. 1998 Nov;24(1):1493-7. 7. Bayramlar H, Unlu C. Incision complications and management. Cataract & Refractive Surgery Management. July 2011. Available at http://crstodayeurope.com/articles/2011-jul/incision-complications-and-management/. Accessed November 28, 2016. 8. Huang Y, Dai Y, Wu X, et al. Toxic anterior segment syndrome after pediatric cataract surgery. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2010;14(5):444-6. 9. Mamalis N, Edelhauser H, Dawson D, et al. Toxic anterior segment syndrome. J Cataract Refract Surg. 2006;32:324-33. 10. Johnston J. Toxic anterior segment syndrome—more than sterility meets the eye. AORN Journal. 2006;84(6):967-83. 11. Lemley C, Han D. Endophthalmitis: a review of current evaluation and management. Retina. 2007;27(6):662-80. 12. Shah R, Garg S. Options to approach retained lens material. Review of Ophthalmology. 2011;18(4):62-5. 13. Sinha R, Shekhar H, Sharma N, et al. Posterior capsular opacification: a review. Indian J Ophthalmol. 2013;61(7):371-6. 14. Aslam T. Systems of analysis of posterior capsule opacification. British J Ophthalmol. 2002;86(10):1181-6. 15. Javitt J, Tielsch J, Canner J, et al. National outcomes of cataract extraction: increased risk of retinal complications associated with Nd;YAG laser capsulotomy. Ophthalmology. 1992;99(10):1487-8. 16. Wright P, Wilkinson C, Balyeat H, et al. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106:740-4. 17. Gass J, Norton E. Follow-up study of cystoid macular edema following cataract extraction. Trans American Academy Ophthalmol Otolaryngol. 1969;73(4):665-82. 18. Henderson B, Kim J, Ament C, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg. 2007;33:1550-8. 19. Kim S, Smiddy W, Feuer W, Shi W. Management of dislocated intraocular lenses. Ophthalmology. 2008;115(10):1699-704. 20. Mönestam E. Incidence of dislocation of intraocular lenses and pseudophakodonesis 10 years after cataract surgery. Ophthalmology. 2009;116(12):2315-20. 21. Campo R, Chung K, Oyakwa R. Pars plana vitrectomy in the management of dislocated posterior chamber lenses. Am J Ophthalmol. 1989;108:529-33. 22. González-Martín-Moro J, González-López J, Gómez-Sanz F, et al. Posterior capsule opacification, capsular bag distension syndrome, and anterior capsular phimosis: A retrospective cohort study. Archivos De La Sociedad Española De Oftalmología (English Edition). 2015;90(2):69-75. 23. Sciscio A, Liu C. Anterior capsular phimosis following Acrysof lens insertion. British J Ophthalmol. 1999;90(2):987. 24. Damgude S, Guliani B. Pseudophakic bullous keratopathy. Photo Essay:41. 25. Taylor D, Atlas B, Romanchuk K, Stern A. Pseudophakic bullous keratopathy. Ophthalmology. 1983;90(1):19-24. |

