Caring for Patients With Brain Injury
More often than not, TBI affects a patient’s vision, and ODs must be prepared to evaluate and manage this population.
By Aaron K. Tarbett, OD
Release Date: July 2017
Expiration Date: July 15, 2020
Goal Statement: Because 75% of patients sustaining a traumatic brain injury (TBI) will experience visual symptoms, eye care providers should be familiar with the basics of TBI and its management. This article provides the foundation of knowledge necessary to alleviate any trepidation practitioners may have in diagnosing and managing this patient population.
Faculty/Editorial Board: Aaron K. Tarbett, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 54095-NO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: The author has no relationships to disclose.
Editorial staff: Jack Persico, Rebecca Hepp, William Kekevian, Michael Riviello and Michael Iannucci all have no relationships to disclose.
A “silent epidemic.”1 That’s how traumatic brain injury (TBI) was described in the 1990s. Today, with conservative estimates of 2.8 million Americans suffering a TBI every year and accounting for 10% of Americans with disabilities, it’s hardly silent anymore.2,3 From sports-related concussion to military TBI, it’s better recognized and often covered in the lay media.4 It’s also more prevalent in optometric practice.
Approximately 50% of the brain’s neural circuitry is dedicated to vision, and 75% of TBI patients will experience visual symptoms.5,6 Given the strong prevalence and impact on vision, optometrists should be familiar with the basics of TBI and its management.
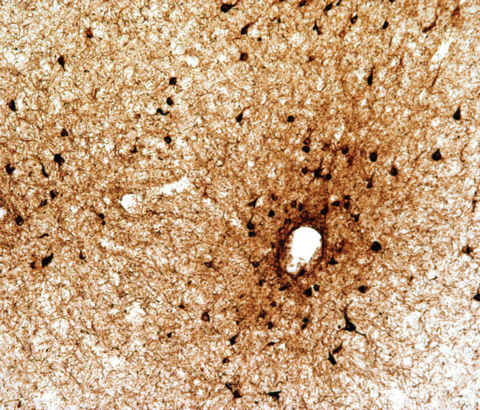 |
| Fig. 1. A focus of perivascular neurofibrillary tangles (black) and neurites at the depth of sulcus in the dorsolateral frontal cortex consistent with stage I/IV CTE, AT8 immunostaining for p-tau, original magnification×100.63. Photo: Ann McKee, MD |
Terminology
The terminology currently employed in brain injury often leads to confusion. The three common terms used to describe brain injury are concussion, mild traumatic brain injury (mTBI) and, most recently, chronic traumatic encephalopathy (CTE). Concussion and mTBI are used interchangeably in medical literature and by medical professionals (as is the case in this article). However, specialists suggest concussion and mTBI should be separate entities with concussion referring to a neurological syndrome involving head trauma and subsequent symptoms, while mTBI is an identifiable injury to brain tissue.7 Currently, this differentiation is difficult, given our common screening and neuroimaging techniques are not sensitive enough to detect such microstructural and physiological changes in the brain.
| Table 1: Glasgow Coma Scale | ||
| Behavior | Response | Score |
| Eye opening response | Spontaneously To speech To pain No response | 4 3 2 1 |
| Best verbal response | Oriented to time, place and person Confused Inappropriate words Incomprehensible sounds No response | 5 4 3 2 1 |
| Best motor response | Obeys commands Moves to localized pain Flexion withdrawal from pain Abnormal flexion to pain (also termed decorticate) Abnormal extension to pain (also termed decerebrate) No response | 6 5 4 3 2 1 |
Classification
Following head trauma, clinical diagnosis and classification of severity is typically done by either the Glasgow Coma Scale (Table 1) or one or a combination of: loss of consciousness (LOC), alteration of consciousness (AOC) such as disorientation or ‘seeing stars’ or post-traumatic amnesia (PTA).8 Both concussion and mTBI are often defined as any LOC or AOC less than 30 minutes or amnesia lasting less than 24 hours (Table 2).4,9 More often than not, concussions are without loss of consciousness and diagnosed by the variable degrees of AOC.10 Any momentary disorientation will qualify as a diagnosis of mTBI, while any identifiable tissue damage or intracranial hemorrhage (subdural, epidural, etc.) on neuroimaging is considered moderate to severe brain injury.11,12
CTE has driven much discussion recently due to its notoriety in sports-related concussion.13 CTE is a progressive, pathological deterioration of the brain caused by repetitive head trauma. It is due only to head trauma with pathognomonic, perivascular accumulations of hyperphosphorylated tau (p-tau) present in neurofibrillary tangles located at the sulcus of brain folds (Figure 1). Current theory speculates the sulci absorb the compressive force of brain movement during injury and consequently accumulate p-tau.14
| Table 2. Severity Rating for TBI | ||||
| Severity | Glasgow Coma Score | Alteration of Consciousness | Loss of Consciousness | Post-traumatic Amnesia |
| Mild | 13-15 | ≤ 24 hrs | 0 to 30 min | ≤ 24 hrs |
| Moderate | 9-12 | > 24 hrs | > 30 min < 24 hrs | > 24 hrs < 7 days |
| Severe | 3-8 | > 24 hrs | ≥ 24 hrs | ≥ 7 days |
Pathophysiology
The pathophysiology in mTBI is complex, not fully understood and involves physiological, structural and vascular processes. Two pathological mechanisms are notable: traumatic axonal injury and microvascular damage.8
Traumatic axonal injury. During a rapid acceleration of or blow to the head, the brain shifts within the skull, causing stretching and twisting of the axons, which are responsible for signal transmission and can course great distances across the brain. With their viscoelastic nature and large surface-to-volume ratio, the axons are fragile and prone to damage. The axolemma, the outer membrane containing ion channels that create membrane potential, is often disrupted, as well as other cytoskeletal elements. Possible axon rupture and excitotoxicty (unchecked neurotransmitter release) follow, leading to an interruption in signal transmission.15,16 Areas commonly affected include the midline regions such as the corpus callosum, brainstem and thalamus—all heavily involved in vision.17
Microvascular damage. Considering the brain contains 400 miles of capillaries, microvascular damage in mTBI is a primary mechanism of tissue injury.18 Pial vessels surround and penetrate brain parenchyma, supplying oxygen and other metabolically needed nutrients. Violent head acceleration can shear these vessels or cause rupture during impact with bony protuberances of the cranium. The decrease in cerebral autoregulatory capacity, in addition to the physical vessel injury, leads to hypoxia, edema and blood toxicity.16,19
 |
| Fig. 2. FL-41 tinted lenses can help patients with brain injury cope with photosensitivity. Photo: Charles Shidlofsky, OD |
Sequelae and Treatment
Patients with acute concussion or mTBI (within one month post-injury) can experience a variety of physical, emotional and cognitive sequelae that will typically resolve without intervention over three months.
However, approximately 15% of patients will have symptoms that persist beyond one year.11,20 This group of patients may retain a number of nonspecific symptoms (fatigue, depression, irritability, etc.) that affect their daily living and quality of life (Table 3). The official ICD-10 diagnosis for this neurological syndrome is postconcussion syndrome (PCS), although patients are anecdotally referred to as the “miserable minority.”21 Here is a look at the most common mTBI sequelae and the treatment options that can help:
Headache. This is the most common symptom following mTBI, and whether the headache is visually related is the primary concern for clinicians.21,22 The most reliable indication of visual involvement is the association with visually related tasks. Although headaches take on many forms following mTBI, migraine and cervical headache are predominant, and these will be managed medically, surgically or with physical therapy.
Clinicians must rule out or treat visual etiologies, while continued management for chronic headache is typically handled by other specialists coordinated by the patient’s primary care provider.
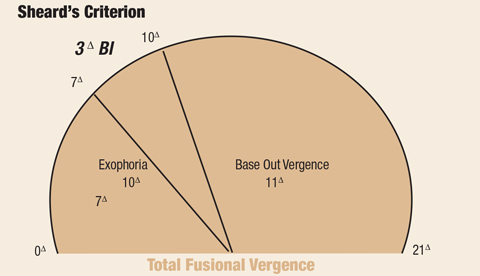 |
| Fig. 3. This criterion can help ECPs diagnose and manage decompensated exophoria. Click image to enlarge. |
Photosensitivity. This remains one of the most common and enigmatic symptoms in PCS. Current theory on the pathophysiology involves intrinsically photosensitive retinal ganglion cells (ipRGC) and their interaction with the thalamus.22 The recent discovery of retinal cells, other than rods and cones, that detect light has caused quite a shift in current theory about the detection of light and the perception of vision.23 Approximately 1% of ganglion cells detect light, thought to be primarily background-related illumination, with the ipRGC axons projecting directly to the thalamus—the primary signal weigh station of the brain that regulates perceptual sensations.24 Brain injury results in thalamic damage and dysregulation of the incoming signal. Principally, upregulation or poor suppression of light intensity (particularly background lighting) results in an enhanced sensitivity to light, and a threshold of pain stimulus is reached as the sensitivity increases.24
Treatment of photosensitivity outdoors logically consists of sunglasses and a brimmed hat; but treatment of debilitating indoor photosensitivity is more controversial. While a patient wearing sunglasses indoors has long been thought to be an indication of non-organic or psychologically related conditions, merit for such a diagnosis may be lacking in the case of brain injury. This patient likely experiences true light sensitivity unrelated to a functional or conversion disorder. However, there is concern that sunglasses for indoor use will establish a “habit-forming tendency” and may actually prevent the resolution of visual or psychological symptoms.25 In this regard, clinicians could recommend titration of tints over a period of time.
A number of studies indicate the usefulness of different tints, such as FL-41 for patients with migraine and blepharospasm (Figure 2).26,27 Interestingly, FL-41 filters 480nm light, the peak activation range of photosensitive retinal ganglion cells.28
| Table 3. Postconcussion Symptoms | ||
| Physical | Cognitive | Emotional |
| Headache Dizziness Fatigue Visual disturbance Noise sensitivity Light sensitivity Insomnia | Memory deficits Attention/concentration deficits Executive function deficits | Irritability Depression Anxiety |
Oculomotor dysfunction. This affects approximately 30% to 50% of mTBI patients at some point.5,29,30 The most common issues are eye tracking (saccades and pursuits), convergence insufficiency and accommodative dysfunction.31,32
Accommodative dysfunction is of particular concern for optometrists, as roughly 50% of pre-presbyopic TBI patients show accommodative dysfunction following TBI vs. 6% in the normal population.33,34 Most clinicians can use Donder’s push up method or minus lenses and address those results with Hofstetter’s formula to assess age-related accommodative ability. Hofstetter’s formula for the minimum amplitude for a given age is defined as: 15 - 1/4 age.35
Reading glasses are an effective treatment since asthenopic symptoms may be short-lived. Considering latent hyperopia is a concern when dealing with accommodative shortages, a cycloplegic refraction is an absolute necessity in those with age-remaining accommodative ability.
Convergence insufficiency (CI). This occurs when the near exophoria overrides the positive fusional vergence ability of the patient to maintain comfortable binocular vision. Research suggests the prevalence of CI in the non-TBI population ranges up to 7.7%, while it can be 42% or higher in the post-TBI population.5,33 The disruption of integrated neurologic circuitry following brain injury reduces the ability to compensate for an existing exophoria. Diagnostic criteria for CI is provided by the Convergence Insufficiency Treatment Trial (CITT) (Table 4).36
Sheard’s criterion, used in the CITT, remains useful in the diagnosis and management of decompensated exophoria (Figure 3).37 It establishes the needed positive fusional vergence (base out) to control exophoria without symptoms. The criterion is defined as the presence of a positive fusional ability twice that of the phoria.38 For example, if a patient presented with near exophoria of 10pd, the positive fusional ability to satisfy Sheard’s criterion would be 20pd.
In addition to determining whether an exophoria should be symptomatically relevant, Sheard’s criterion also provides a recommendation for judicious use of prism in patients with CI. Prism is a recommended treatment practice, particularly considering postconcussion CI symptoms could be transitory.39 According to Sheard’s criterion, the needed prism to help carry the load of the overburdened vergence system will be equal to the amount of prism to place a patient’s phoria within a third of their overall vergence ability. For example, a patient with 10pd exophoria and 11pd base-out vergence at near would need 3pd of base-in prism applied to their spectacles, moving the image toward apex.
This formula will also provide the practitioner with the recommended prism: 2/3(phoria) - 1/3(positive fusional vergence).36
Resultant prism from Sheard’s calculations should be trial framed for patient approval. However, Sheard’s criterion is only a recommendation, and they may be successful with less prism than indicated.
Small studies and anecdotal experience attest to the effectiveness of vision therapy/rehabilitation as a treatment modality for TBI-related visual symptoms.40 However, without large scale, prospective studies, it is not a universally recommended treatment; clinicians should first consider a brief trial of vision rehabilitation. The 2016 Department of Defense and Veterans Affairs guidelines for traumatic brain injury issues a word of caution for all rehabilitative therapies, including vision rehabilitation.39 A prolonged course of therapy (three to six months) without significant improvement will considerably influence a patient’s perception of their ability to recover from a TBI, fostering negative expectations.41,42
| Table 4. CITT Convergence Insufficiency Criteria Near exophoria at least 4pd greater than at distance Plus Additional Signs: |
|
Dizziness, disequilibrium and imbalance. These commonly occur after mTBI and are an indicator of a potentially long recovery process in PCS.43,44 Vestibular dysfunction, both peripheral (inner ear) and central (cerebellar), is a common etiology. After ruling out obvious oculomotor deficiencies, (e.g., muscle palsies, decompensated phorias and especially mild vertical phorias) impairment of the vestibulo-ocular reflex (VOR) is one of the leading concerns for optometrists. The vestibulo-ocular system is responsible for gaze stability (non-pursuit) during high frequency and velocity-related head movements. When the VOR is disrupted, oscillopsia and dizziness ensue.45 Its counterpart, the vestibulospinal system, is responsible for postural control with consequences of disrupted balance.46,47 The integrity of the vestibulo-ocular system can be evaluated by assessing VOR with dynamic visual acuity (DVA).
When performing DVA testing, clinicians establish a baseline with best-corrected visual acuity and then rotate the head from side to side, managing one complete cycle (left, right and back left) with two cycles per second. At this rate, VOR dysfunction may exist if the patient reads worse than two lines below their baseline acuity.48
Research suggests vestibular therapy that involves gaze stabilization exercises is effective in these patients.49 It is common to work with an interdisciplinary team comprised of neurology, ENT, audiology and physical therapy with patients in need of vestibular rehabilitation.
Visuospatial disruptions in TBI are more perplexing, since they are higher-order dysfunctions of the brain and are poorly understood. A commonly encountered symptom in mTBI is increased visual motion sensitivity (VMS). With increased VMS, a patient will report imbalance, nausea and disorientation in situations with significant peripheral motion such as walking through a shopping mall or riding in a car.50
Research shows binasal occlusion (BNO) is an effective treatment, both subjectively and objectively. Clinicians can add partial occluders to the patient’s spectacle lenses to suppress excessive visual motion in the peripheral visual field. The occlusion can be done using scotch tape, bangerter foils or electrical tape placed nasal to the pupillary-limbal margin and oriented with a 15° superior-temporal tilt (Figure 4).51,52
Patients with TBI may also experience a shift in their perception of midline. Known as abnormal egocentric localization, visual midline shift or optic ataxia (misreaching to visual targets), it can cause a patient to lean to one side, forward or backward, resulting in difficulties with balance during ambulation.
To diagnose and manage these shifts, clinicians must first determine where the shift occurs (right, left, forward or backward) and then attempt to correct the misperception with prism.53,54 For example, a patient notes when you hold a pen directly in front of them that it appears to the left of center. Shifting the patient’s midline back toward the center with base left yoked prism (approximately 2pd to 6pd) may help correct the perceived imbalance. Similarly, if the patient tends to lean forward, yoked base down prism (also 2pd to 6pd, but could vary depending on the case) could be trialed for improvement in posture.
 |
| Fig. 4. Binasal occlusion can help TBI patients overcome visual motion sensitivity in the peripheral visual field. Photo: Marc Taub, OD |
TBI and PTSD
Post-traumatic stress disorder (PTSD) is frequently associated with TBI. While approximately 50% of military service members that have sustained an mTBI have associated PTSD, it is not unique to military members. Those who have sustained an mTBI from motor vehicle accidents also have a 50% chance of developing PTSD.55,56 Patients with more violent or anxiety-laden brain injury events logically have higher rates of PTSD development.
Current theory suggests damage to the rational thinking medial prefrontal cortex (mPFC) causes an inability to regulate the fear response. Especially damaging is the evolutionary juxtaposition of the amygdala, the center of the brain for fear, and the hippocampus, the region for memory development (Figure 5). Situated next to each other, without mPFC tempering, the exaggerated fear response becomes inextricably linked with a memory, thus generating the associated PTSD symptoms.57
Post-concussion symptoms such as headache, lack of concentration and poor sleep, among others, may be more related to PTSD than PCS.58 Although the high rates of anxiety and depressive disorders related to PTSD exacerbate visual symptoms, no studies indicate visual symptoms are directly linked to PTSD.
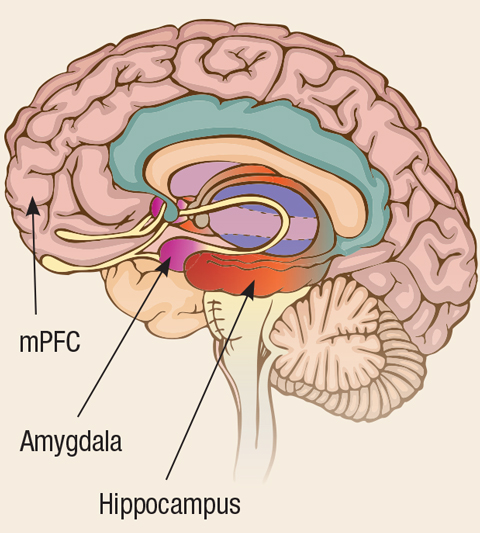 |
| Fig. 5. Because the amygdala and the hippocampus are situated next to each other, the exaggerated fear response becomes inextricably linked with a memory, creating symptoms of PTSD. |
Malingering and Stereotype Threat
Managing a patient with TBI usually goes beyond clinical medicine. The social and psychological ramifications of brain injury can lead to other complications such as malingering and stereotype threat.
Malingering. This is a major dilemma with mTBI since it is difficult to tell if symptoms are truly related to injury. Survey data suggest 20% to 40% of mTBI patients present with exaggerated or fabricated neuropsychological deficits.59 A number of complicating factors exist with TBI-related malingering, such as the difficult distinction between pre-TBI abilities and post-TBI deficits. Researchers found that, for many patients, complaints following mTBI were more a reflection of a pre-TBI condition that was brought to the fore by the TBI.60,61
There is also the matter of volition, or the degree to which a behavior is both conscious and deliberate. Optometrists should look to neuropsychological personnel to assess an individual’s volition in malingering and other disorders. In addition, malingering and legitimate neurologic deficit from concussion are not mutually exclusive, possibly compelling providers to attempt to manage nonorganic or fictitious ailments.
Stereotype Threat. This social psychology theory purports that a stereotyped individual, when placed in a situation that risks validation of that stereotype, performs poorly due to “performance pressure,” thus supporting the stereotype.62
Stereotype threat is a proven phenomenon in mTBI patients, as society does hold specific maladaptive beliefs regarding the cognitive effects of brain injury.61 Thus, patients diagnosed with mTBI may do poorly on cognitive or memory testing simply because they have been diagnosed with mTBI, not due to any associated sequelae.
Optometrists must be aware of this during the exam but, more importantly, on treatment recommendations and patient interaction. Stigmatizing or fostering negative expectations for patients with prolonged courses of ineffective treatment or communicating with a despondent bedside manner could set patients back substantially in recovery.
With a high prevalence and an increasing community awareness of brain injury, it is not a question of whether optometrists will see these patients, but when. Having an adequate foundation of understanding will alleviate any trepidation and mystique in managing them.
Dr. Tarbett is the former chief of the Optometry Service at Walter Reed Army Medical Center. He is currently on staff at the Hefner VAMC in Salisbury, NC.
| 1. Goldstein M. Traumatic brain injury: a silent epidemic. Ann Neurol. 1990;27(3):327. 2. Centers for Disease Control and Prevention. Get the stats on traumatic brain injury in the United States. www.cdc.gov/traumaticbraininjury/pdf/bluebook_factsheet-a.pdf. Accessed April 13, 2017. 3. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths-United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1-16. 4. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. 2015. 5. Brahm KD, Wilgenburg HM, Kirby J, et al. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom Vis Sci. 2009;86(7):817-25. 6. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1-47. 7. Keener AB. Tackling the brain: Clues emerge about the pathology of sports-related brain trauma. Nat Med. 2016;22(4):326-9. 8. Kushner D. Mild traumatic brain injury: toward understanding manifestations and treatment. Arch Intern Med. 1998;158(15):1617-24. 9. Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87. 10. Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282(10):964-70. 11. Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004 Feb;(43 Suppl):84-105. 12. Narayan R, Wilberger, JE, Povlishock, JT, eds. Neurotrauma. New York, NY: McGraw-Hill; 1996. 13. Fainaru-Wada M, Avila J, Fainaru S. Doctors: Junior Seau's brain had CTE. January 11, 2013. www.espn.com/espn/otl/story/_/id/8830344/study-junior-seau-brain-shows-chronic-brain-damage-found-other-nfl-football-players. Accessed April 20, 2017. 14. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43-64. 15. Bauer D, Tung ML, Tsao JW. Mechanisms of traumatic brain injury. Semin Neurol. 2015;35:e14-22. 16. Choe MC. The pathophysiology of concussion. Curr Pain Headache Rep. 2016;20:42. 17. Grossman EJ, Inglese M. The role of thalamic damage in mild traumatic brain injury. J Neurotrauma. 2016;33(2):163-7. 18. Cipolla M. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2009. 19. Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg. 1997;86(3):425-32. 20. Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):1253-60. 21. Ruff RM, Crouch JA, Troster AI, et al. Selected cases of poor outcome following a minor brain trauma: comparing neuropsychological and positron emission tomography assessment. Brain Inj. 1994;8:297-308. 22. Noseda R, Constandil L, Bourgeais L, et al. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30(43):14420-9. 23. Xue T, Do MT, Riccio A, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479(7371):67-73. 24. Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32(1):68-81. 25. Truong JQ, Ciuffreda KJ, Han MH, Suchoff IB. Photosensitivity in mild traumatic brain injury (mTBI): a retrospective analysis. Brain Inj. 2014;28(10):1283-7. 26. Good PA, Taylor RH, Mortimer MJ. The use of tinted glasses in childhood migraine. Headache. 1991;31(8):533-6. 27. Blackburn MK, Lamb RD, Digre KB, et al. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmol. 2009;116(5):997-1001. 28. Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4(6):621-6. 29. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702. 30. Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. Am J Sports Med. 2012;40(10):2375-84. 31. Master CL, Scheiman M, Gallaway M, et al. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila). 2016;55(3):260-7. 32. Goodrich GL, Kirby J, Cockerham G, et al. Visual function in patients of a polytrauma rehabilitation center: A descriptive study. J Rehabil Res Dev. 2007;44(7):929-36. 33. Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997;74(2):111-3. 34. Stelmack JA, Frith T, Van Koevering D, et al. Visual function in patients followed at a Veterans Affairs polytrauma network site: an electronic medical record review. Optometry. 2009;80(8):419-24. 35. Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkens; 2008. 36. Convergence Insufficiency Treatment Trial Study G. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126(10):1336-49. 37. Gray L. The prescribing of prisms in clinical practice. Graefes Arch Clin Exp Ophthalmol. 2008;5(5):627-9. 38. Sheard C. Zones of ocular comfort. Am J Optom. 1930;7:9-25. 39. Heinmiller L, Gunton KB. A review of the current practice in diagnosis and management of visual complaints associated with concussion and postconcussion syndrome. Curr Opin Ophthalmol. 2016;27(5):407-12. 40. Thiagarajan P, Ciuffreda KJ. Effect of oculomotor rehabilitation on accommodative responsivity in mild traumatic brain injury. J Rehabil Res Dev. 2014;51(2):175-91. 41. VA/DoD Clinical Practice Guideline for the Management of Concussion-mild Traumatic Brain Injury. 2016. www.guideline.gov/summaries/summary/50140/vadod-clinical-practice-guideline-for-the-management-of-concussionmild-traumatic-brain-injury. Accessed March 29, 2017. 42. Kay T. Neuropsychological treatment of mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 1993;8:74-85. 43. Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87-93. 44. Ellis MJ, Cordingley D, Vis S, et al. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2015;16(3):248-55. 45. Grossman GE, Leigh RJ, Abel LA, et al. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70(3):470-6. 46. Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35(3):185-96. 47. Khan S, Chang R. Anatomy of the vestibular system: a review. NeuroRehabilitation. 2013;32(3):437-43. 48. Rakozcy C. Brain Injury Electronic Resource Manual: Vol 1 A: Traumatic Brain Injury. American Optometric Association. 2013. 49. Hall CD, Heusel-Gillig L, Tusa RJ, Herdman SJ. Efficacy of gaze stability exercises in older adults with dizziness. J Neurol Phys Ther. 2010;34(2):64-9. 50. Winkler PA, Ciuffreda KJ. Ocular fixation, vestibular dysfunction, and visual motion hypersensitivity. Optometry. 2009;80(9):502-12. 51. Padula WV, Argyris S, Ray J. Visual evoked potentials (VEP) evaluating treatment for post-trauma vision syndrome (PTVS) in patients with traumatic brain injuries (TBI). Brain Inj. 1994;8(2):125-33. 52. Yadav NK, Ciuffreda KJ. Effect of binasal occlusion (BNO) and base-in prisms on the visual-evoked potential (VEP) in mild traumatic brain injury (mTBI). Brain Inj. 2014;28(12):1568-80. 53. Ciuffreda KJ, Ludlam DP, Yadav NK, Thiagarajan P. Traumatic brain injury: visual consequences, diagnosis, and treatment. In: Advances in Ophthal and Optom. Yanoff M, ed. Philadelphia: Elsevier; 2016:307-33. 54. Padula WV, Nelson CA, Padula WV, et al. Modifying postural adaptation following a CVA through prismatic shift of visuo-spatial egocenter. Brain Inj. 2009;23(6):566-76. 55. Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-63. 56. Mayou RA, Ehlers A, Hobbs M. Psychological debriefing for road traffic accident victims. Three-year follow-up of a randomised controlled trial. Br J Psychiatry. 2000;176:589-93. 57. Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60(4):376-82. 58. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167:1446-52. 59. Dominic Carone DA, Bush SS, eds. Mild Traumatic Brain Injury: Symptom Validity Assessment and Malingering. New York, NY: Springer Publishing Company; 2012. 60. Greiffenstein FM, Baker JW. Comparison of premorbid and postinjury mmpi-2 profiles in late postconcussion claimants. Clin Neuropsychol. 2001;15(2):162-70. 61. Kit KA, Mateer CA, Tuokko HA, Spencer-Rodgers J. Influence of negative stereotypes and beliefs on neuropsychological test performance in a traumatic brain injury population. J Int Neuropsychol Soc. 2014;20(2):157-67. 62. Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. J Pers Soc Psychol. 1995;69(5):797-811. 63. McKee, et al. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. |
