 |
Can You Spot these Drug-induced Ocular Side Effects?
Consider these four cases involving toxic optic neuropathy and maculopathy to learn more about the potential mishaps of systemic meds.
By Sweta Das, OD
Jointly provided by the Postgraduate Institute for Medicine (PIM) and the Review Education Group
Release Date: September 15, 2024
Expiration Date: September 15, 2027
Estimated Time to Complete Activity: two hours
Target Audience: This activity is intended for optometrists interested in effectively addressing DED and ensuring optimal outcomes.
Educational Objectives: After completing this activity, participants should be better able to:
Evaluate and identify various ocular side effects associated with systemic agents.
Recognize toxic optic neuropathy and the potential for structural changes after drug use.
Effectively use diagnostic tests to identify changes in the retina and optic nerve due to systemic medications.
Determine appropriate management strategies for drug-induced ocular side effects.
Disclosure of Conflicts of Interest: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in health care and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Faculty - Dr. Das has no financial disclosures. Planners and Editorial Staff - PIM has nothing to disclose. The Review Education Group has nothing to disclose.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Credit Statement: This course is COPE-approved for two hours of CE credit. Activity #129131 and course ID 93294-PH. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure of Unlabeled Use: This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings.
Disclaimer: Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s condition(s) and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information and comparison with recommendations of other authorities.
Optometrists play a critical role in not only managing vision but also safeguarding overall eye health. With the increasing prevalence of systemic medications that can affect the eyes, being able to identify drug-induced ocular side effects is more important than ever. From dry eyes to vision-threatening conditions, a wide range of medications can cause adverse effects that may be subtle yet significant.
Our goal is not only to accurately diagnose structural changes occurring in our patients’ eyes but also to manage the condition appropriately to mitigate functional visual deterioration. This article will discuss the typical features of toxic optic neuropathy (gradual bilateral symmetric painless decrease in vision, cecocentral or central visual field loss, and decrease in color vision) and highlight a case of toxic optic neuropathy secondary to oral ciprofloxacin use.1
Toxic optic neuropathy selectively affects the papillomacular bundle, but, rarely, maculopathies, like hydroxychloroquine-related retinal toxicity, may cause changes in the macula region nasal to the fovea and mimic the structural changes seen in toxic optic neuropathy.2,3 This discussion will highlight hydroxychloroquine (HCQ)-related retinal toxicity that mimics neurogenic etiologies.3 An antibiotic drug minocycline is known to cause discoloration of tissue (skin, conjunctiva and sclera), but this article will discuss pigmentary and structural changes in retinal pigmentary epithelial (RPE) cells secondary to oral minocycline use.4 Additionally, it will touch upon a case of serous retinal detachment from immune dysregulation after the use of immune checkpoint inhibitors for treatment of metastatic cancer.5
Equipped with the knowledge to recognize potential issues early, optometrists can provide the best care for their patients, supporting their health and well-being as a whole.
 |
|
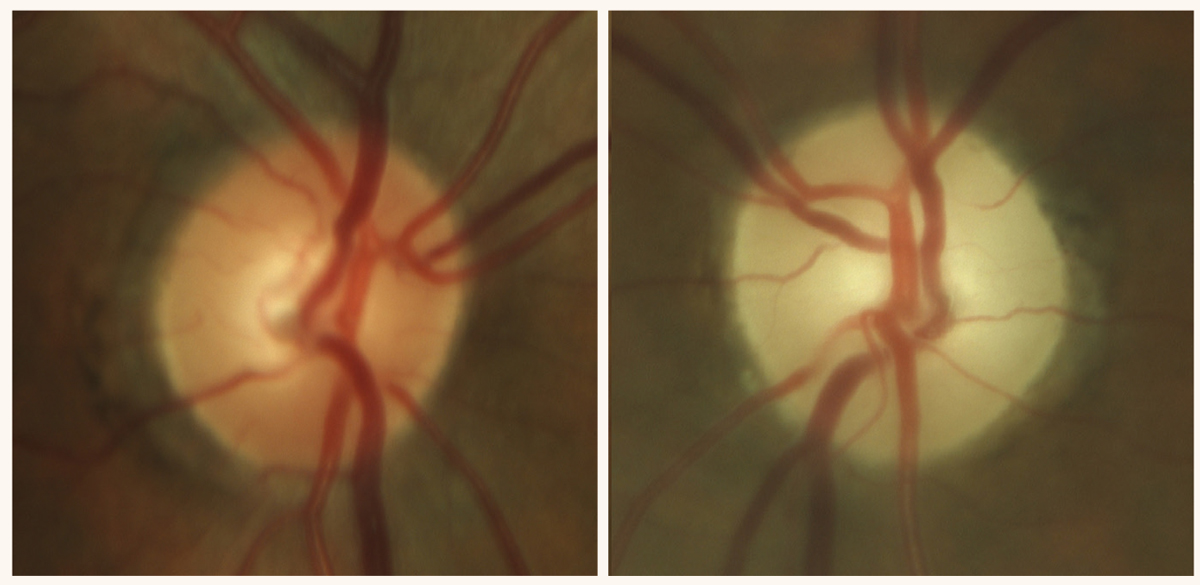
Late-stage, severe optic atrophy in a patient on chronic amiodarone therapy, an anti-arrhythmic drug. Photo: Sara Weidmayer, OD. Click image to enlarge. |
Toxic Optic Neuropathy
Some of the ocular side effects of oral ciprofloxacin—an antibacterial drug that belongs to a class of drugs called fluoroquinolones—are eyelid irritation and retinal detachment. Oral use of moxifloxacin is associated with iris transillumination defects and retinal detachment, and fluoroquinolones in general are also associated with exacerbation of myasthenia gravis. One of the lesser-known side effects of oral ciprofloxacin use, as discussed below, is optic neuropathy.
One study reported a case of a 55-year-old male who experienced bilateral progressive loss of vision and color vision along with central scotoma over a two-month period from toxic optic neuropathy.1 He reported difficulty recognizing faces and reading.
Case history. His ocular history was unremarkable and medical history was remarkable for chronic osteomyelitis managed with oral ciprofloxacin 1,500mg per day and opioid analgesics for the last six years. The patient also reported intermittent use of cephalexin for recurrent urinary tract infection. His social history included variable use of alcohol intake, but the patient denied intake of alcohol in the last four months.
Pertinent findings. The patient’s corrected visual acuity was reduced bilaterally to 6/60 [20/200] in each eye. His visual acuity recorded 12 months prior was 6/6 [20/20] in each eye. Color vision measured with Ishihara color plates (IHP) was 3/15 in each eye. His pupils were equally round and reactive to light without a relative afferent pupillary defect. The optic nerves did not show any indication of edema or pallor. A 24-2 Humphrey visual field (HVF) test showed bilateral central scotoma.
Additional tests. Computed tomography and gadolinium-enhanced magnetic resonance imaging (MRI) of the brain and orbits did not reveal a compressive lesion and the result was unremarkable. Laboratory tests to evaluate for inflammatory, infectious, vascular and hereditary causes were unremarkable. Of note were elevated gamma glutamyl transferase levels and macrocytic anemia, which raised suspicion of alcohol abuse but his normal levels of vitamin B12, folate and thiamine did not support the diagnosis of alcohol abuse.
 |
|
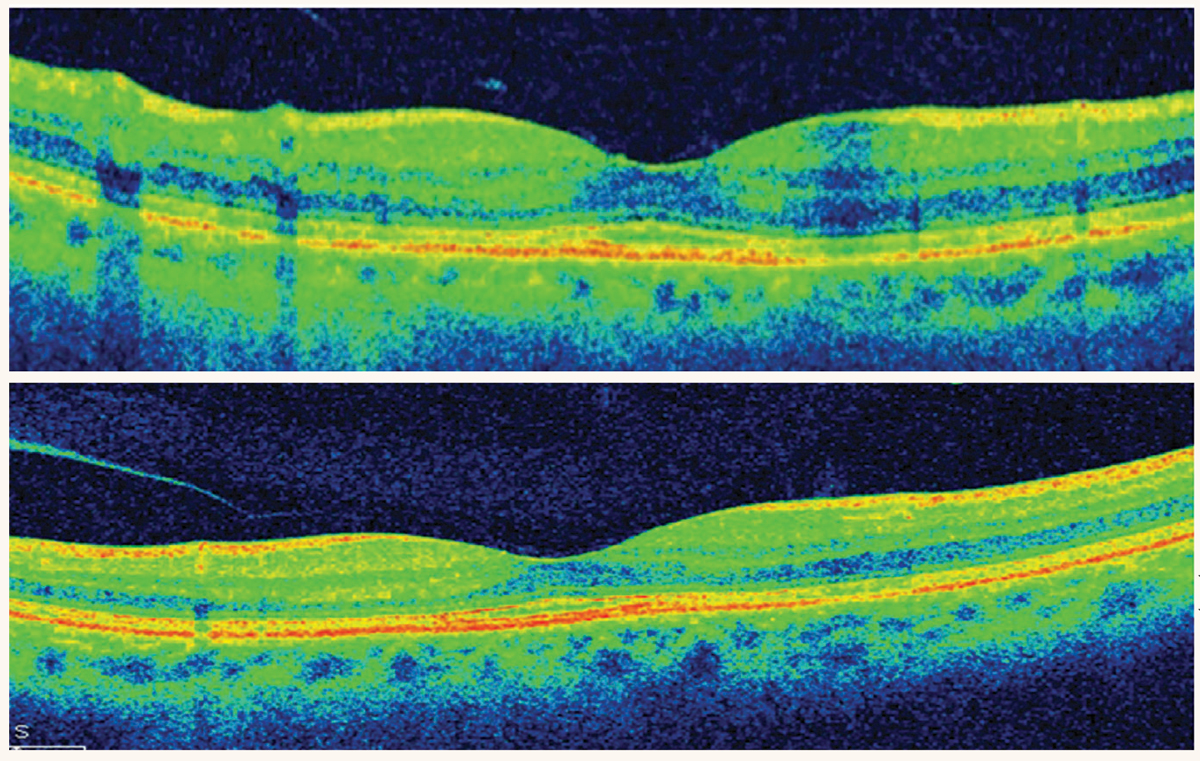
OCT demonstrating localized parafoveal thinning in a patient with early HCQ toxicity. Photo: Marlon Demeritt, OD, Sherrol Reynolds, OD, Diana Shechtman, OD, and Jennifer Davidson, OD. Click image to enlarge. |
Diagnosis and management. This patient was diagnosed with ciprofloxacin-induced toxic optic neuropathy and was switched to oral cephalexin 500mg four times a day for the management of chronic osteomyelitis. He was asked to discontinue the use of oral ciprofloxacin 1,500mg per day.
Prognosis. At the three-month follow-up exam after cessation of oral ciprofloxacin, visual acuity improved to 6/30 [20/100] in each eye. There was further improvement in visual acuity at a 36-month follow-up exam to 6/6 [20/20] in the right eye and 6/12 [20/40] in the left eye. Visual field showed improvement in both eyes with only a shallow central scotoma seen in the left eye’s field. Even though the patient’s visual acuity and field improved after cessation of oral ciprofloxacin, color vision remained affected at 3/15 with IHP with each eye, and the patient developed bilateral sectoral optic disc pallor and optic nerve atrophy.
Discussion. As clinicians, it is important to determine the speed at which our patient experiences a decrease in vision or loss of vision. When a patient presents with sudden onset vision loss without subsequent progression, it is likely that the culprit is an ischemic insult such as an arterial occlusion. Patients with ischemic optic neuropathy may complain of sudden onset of vision loss with subsequent progression in loss of vision over days to weeks. Loss of vision that occurs gradually over days to weeks indicates an inflammatory, infectious or demyelinating cause, whereas gradual decrease in vision progressing over months to years is suspicious for a compressive lesion, nutritional/toxic optic neuropathy or glaucomatous optic neuropathy, but it is crucial that we keep atypical maculopathies in the differential. The pathognomonic feature of toxic/nutritional optic neuropathy is gradual progressive bilateral symmetric painless loss of vision, loss of color vision and symmetric central or cecocentral loss of visual field. Improvement in visual function but often not in structural change (retinal nerve fiber layer/ganglion cell-inner plexiform layer thinning and optic nerve pallor) after discontinuation of the offending agent is also a common characteristic of toxic optic neuropathy.2 Table 1 lists agents that are known to induce toxic optic neuropathy.6
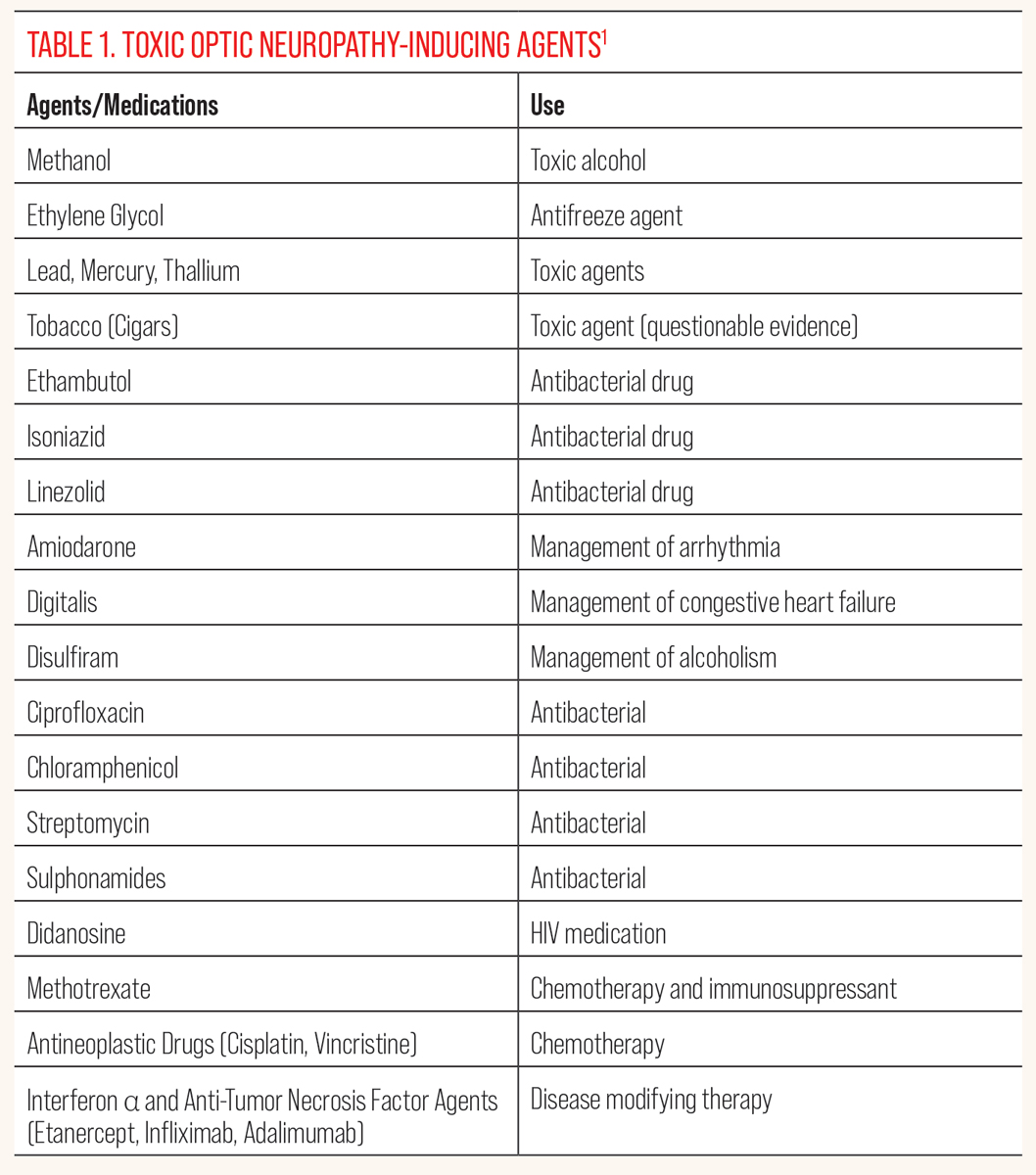 |
| Click table to enlarge. |
Long-standing use of oral ciprofloxacin can lead to toxic optic neuropathy; therefore, it is prudent to monitor patients, who are on long-term oral ciprofloxacin, yearly with a comprehensive dilated eye exam, color vision, fundus photos and visual field tests. Discontinuation of oral ciprofloxacin on a timely basis can lead to resolution of central scotoma and near normalization of visual field slowly over months but does not reverse development of optic disc pallor and structural changes (retinal nerve fiber layer and ganglion cell-inner plexiform layer thinning).
Is this Retinopathy or Neuro-Ophthalmic Disease?
HCQ is a chloroquine derivative that is used in the management of rheumatologic and dermatologic conditions and is less likely to cause retinal toxicity than chloroquine. Even though the incidence rate of HCQ-related retinopathy is low, there are well-documented cases of retinopathy in patients on HCQ, not exceeding the maximum recommended daily dose of 5mg/kg real body weight/day and in the absence of associated risk factors for developing drug-induced toxicity.3,7,8
Risk factors known to increase the risk for HCQ-related retinal toxicity include cumulative total dose of HCQ greater than 1,000g, greater than five years of HCQ intake, presence of kidney disease, concomitant use of tamoxifen and presence of retinal disease, such as age-related macular degeneration (AMD). HCQ is known to bind to melanin in RPE. Rare but irreversible manifestations of HCQ-related retinal toxicity includes damaged photoreceptors and underlying RPE parafoveally, which we know as the classic bullseye maculopathy. If drug-related retinal toxicity is not diagnosed early on and the patient continues the use of HCQ, retinal pigmentary changes may progress to peripheral retinal degeneration that appear similar in presentation as retinitis pigmentosa. There are case reports of early-onset pericentral and peripheral retinopathy, but HCQ-related retinopathy may even be localized nasal to the fovea in a peripapillary pattern.
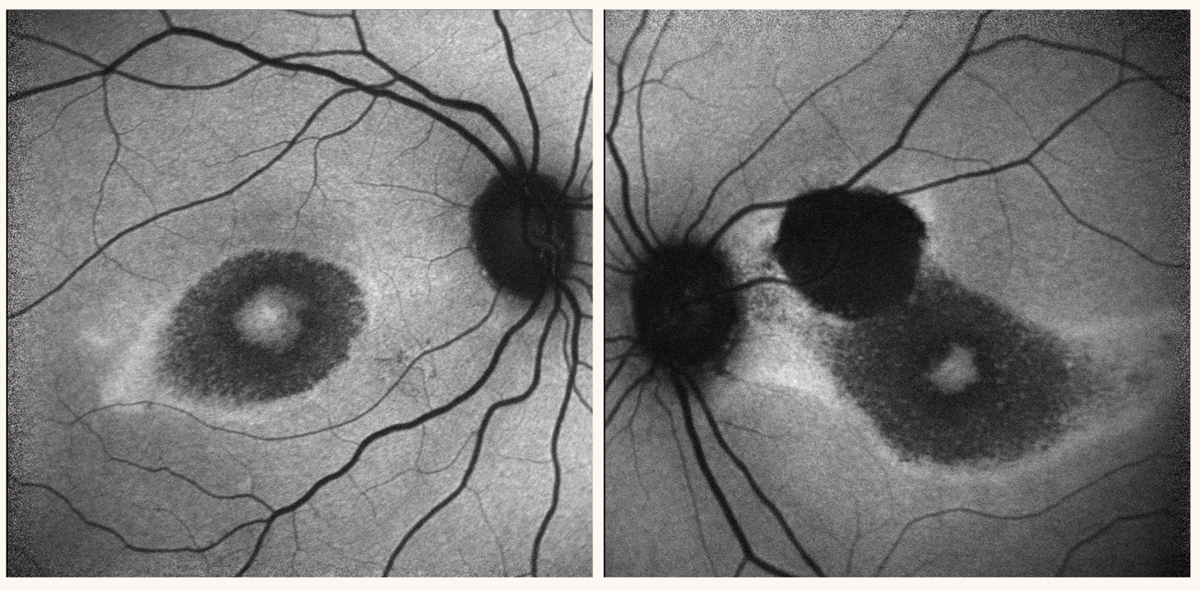
Case report of an atypical manifestation of HCQ-related retinopathy. One study shared a series of case reports highlighting atypical manifestations of HCQ associated retinopathy.3 A 54-year-old Korean female from the case series reported intake of HCQ for approximately six years for Sjögren’s syndrome, whose 30-2 HVF result was remarkable for bitemporal central sparing hemianopsia. Given the bitemporal nature of the visual field defect, a brain MRI was performed without remarkable findings. Her ultrawide fundus autofluorescence (FAF) showed bilateral peripapillary and pericentral (involving superior and inferior vascular arcade region) hypoautofluorescence. Macular OCT scan showed loss of ellipsoid, interdigitation zone and damage to the RPE and pigment migration pericentrally (along vascular arcade).
Diagnosis and management. The center-sparing bitemporal hemianopsia corresponding with bilateral nasally localized retinopathy was recognized as HCQ-associated retinal toxicity in this case series.3 Acute macular neuroretinopathy is a rare condition, but it may also present as bilateral disruption of the ellipsoid zone, nasal to the fovea, on macular OCT, and therefore it is worth keeping in the differentials.6
Discussion. HCQ-associated retinopathy may be localized nasally and may lead to bitemporal hemianopsia. This case report highlighted the importance of expanding our differential diagnosis for bitemporal hemianopsia from chiasmal disorders to medication induced retinal toxicity.3 The abnormal hypoautofluorescence seen nasally in both eyes, in addition to the corresponding outer retinal layer disruption seen on macular-OCT in the setting of normal brain MRI, confirms the diagnosis of HCQ-associated retinal toxicity.
 |
|
Perifoveal hypoautofluorescence in both eyes and a dense hypoautofluorescent lesion in the superonasal macula consistent with the chorioretinal scar observed on fundus exam. Photo: Rami Aboumourad, OD, and Kalie Leone, OD. Click image to enlarge. |
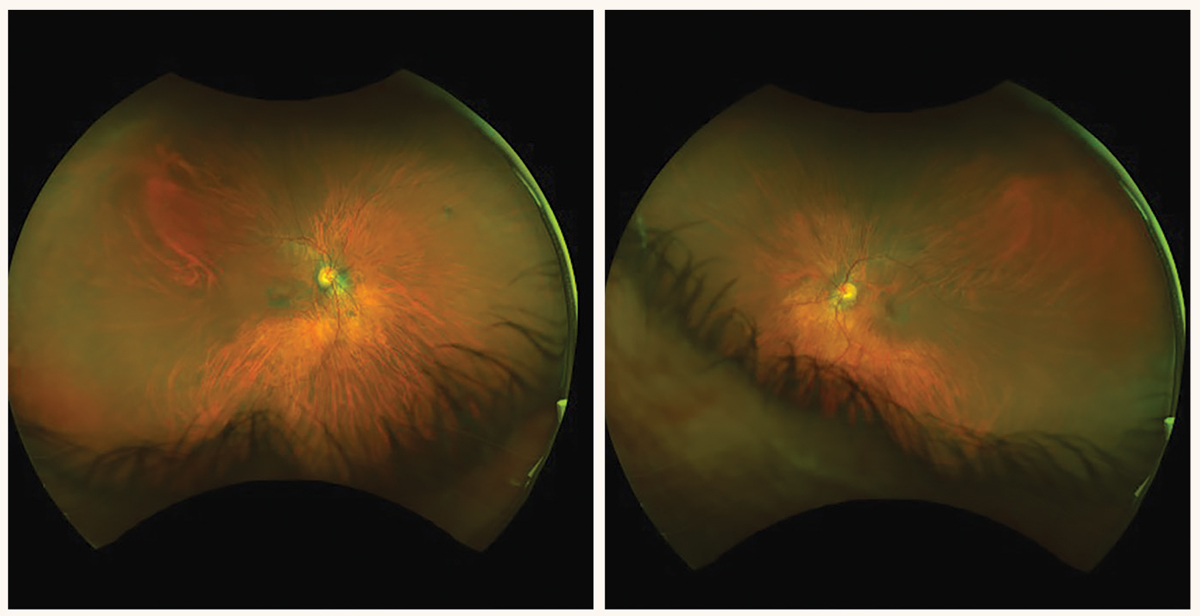
The researchers also reported a case of retinal toxicity in a 44-year-old female after being on unknown daily dose of HCQ for as early as six months without any of the risk factors like kidney disease, concomitant use of tamoxifen, presence of retinal disease, cumulative total dose of HCQ greater than 1,000g or duration of HCQ intake for longer than five years.3 They also highlighted a case of HCQ-related retinopathy that started out in the peripheral retina in both eyes as opposed to parafoveal or pericentral regions, which are the most commonly affected. Yet another interesting case reported was HCQ retinopathy noted in one eye of a patient, while the contralateral eye remained unaffected.
Even though parafoveal and pericentral outer retinal layer disruption is considered pathognomonic features of HCQ-associated retinopathy, we must be cognizant of peripapillary, peripheral, asymmetric or early-onset outer retinal changes secondary to HCQ-related retinal toxicity. This also means that patients who are on HCQ may benefit from screening every six to 12 months with not only a careful macular evaluation but also a thorough evaluation of the peripheral retina. Macular OCT, HVF 10-2 and/or fundus photos are commonly administered for patients on HCQ as part of their retinal evaluation. Ultra-widefield (UWF) FAF and color photos along with HVF 24-2 or HVF 30-2 may be beneficial, so we do not miss peripheral or pericentral retinal changes from HCQ-related toxicity.3 As primary eyecare providers, it is crucial that we are aware of the atypical presentation of HCQ-associated retinal toxicity in order to prevent this iatrogenic cause of progressive and irreversible vision loss.
Pigmentary and Structural Change in RPE
Discoloration of tissue (skin, nail, teeth), sclera and conjunctiva is a widely known side effect of a synthetic tetracycline antibiotic, known as minocycline hydrochloride.9,10 Pigmentary and structural change of RPE cells is a lesser-known adverse effect of oral minocycline.10 Minocycline agent is commonly used to treat rosacea, acne vulgaris, bullous pemphigoid and rheumatoid arthritis on a long-term basis.10
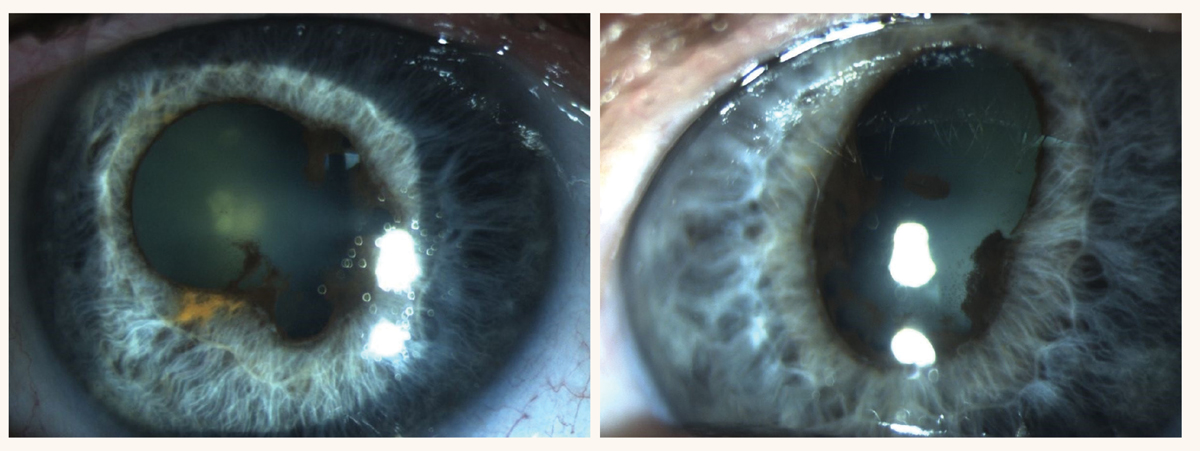
Case reports. One study reported a case of a patient in their early 70s on prolonged use of oral minocycline (100mg twice a day for over 30 years) for acne vulgaris, who presented with blue-gray discoloration of sclera and bluish-gray conjunctival inclusions in inferior palpebral conjunctiva.4 His visual acuity was 20/20 in the right eye and 20/30 in the left eye secondary to nuclear sclerosis, more significant in the left eye. There were also dark gray pigment changes noted in the macula region of both eyes. On spectral-do OCT scan of the macula, these dark pigment deposits appeared as sub-RPE hyper-reflective material, which did not show abnormal hyper- or hypofluorescence on fluorescein angiography. The patient had no visual complaints and reported no metamorphopsia.
 |
|
Five years after this patient stopped taking HCQ, these fundus images highlight some of the damage it left behind. Note the incomplete bullseye maculopathy at left. Photo: Luanne Chubb, OD. Click image to enlarge. |
Diagnosis and management. Bilateral darker macular pigmentation noted on ophthalmoscopic examination did not resemble the appearance of drusen, and the nodular hyperreflective sub-RPE deposits noted on the macular OCT bilaterally were diagnosed as minocycline-induced pigmentary and structural change in RPE. Even though the patient did not complain of visual distortion, cessation of minocycline was recommended due to subfoveal pigmentary and structural change noted in RPE of both eyes. It was also suggested that the patient be followed closely with eye exam to further assess for ocular and visual changes in the future. A dilated fundus examination supplemented with fundus photographs, macular OCT and central 10-2 visual field test is usually sufficient in careful monitoring of patients with minocycline-induced retinal pigmentary and structural changes. Macula appears normal (hypoautofluorescent) on fundus autofluorescence indicating low lipofuscin content but rather melanin-associated RPE changes instead.11
Discussion. Minocycline is a yellow lipophilic substance that turns black upon oxidation. It is known to bind various proteins in the body like ferritin, hemosiderin and melanin.3 This oxidized drug complex with various proteins in the body is the cause for discoloration of the skin, teeth, bone, sclera and conjunctiva. Studies suggest that subfoveal RPE cells have the highest concentration of melanin and is believed to be the cause for pigmentary and structural change of RPE in the macula region. Even though cessation of minocycline leads to resolution of discoloration noted elsewhere in the body without functional alteration of the organ in question, discontinuation of minocycline use and its long-term effect on pigmentary and structural change of RPE is not well established yet.
Long-term use of oral minocycline can not only cause blue-gray discoloration of skin, teeth, sclera and palpebral conjunctiva but also of the macula, specifically the subfoveal RPE cells. This nodular deposit appears as sub-RPE hyperreflective material in the fovea region and can resemble drusen (commonly seen in nonexudative macular degeneration) on OCT scan of the macula. However, ophthalmoscopic exam shows minocycline-induced dark pigmentary change in the macula instead of drusen. Therefore, it may be beneficial for optometrists to keep minocycline-induced RPE change as one of our differentials when macular OCT suggest drusenoid sub-RPE deposit without corresponding appearance of drusen in the macula ophthalmoscopically.
Structural Change of Photoreceptor Outer Segment and Choroid
Immune checkpoint inhibitors are used for managing advanced cancer, including malignant melanoma, renal carcinoma and Hodgkin lymphoma. The mechanism of action includes preventing cancer cells from growth by stimulating T-cell activation. Immune checkpoint inhibitors are associated with autoimmune-mediated complications; ocular complications reported include dry eye, Vogt-Koyanagi-Harada disease and uveitis. Below is a case reported involving multiple bilateral serous retinal detachments, thickening of photoreceptor outer segments and choroid after treatment with nivolumab (immune checkpoint inhibitor) for stage 4 malignant nasal melanoma.5
 |
|
Photo: Sara Weidmayer, OD. Click image to enlarge. |
Case report. A 73-year-old Japanese man was seen for an eye exam due to complaints of bilateral metamorphopsia, which developed two months after he was started on the immune checkpoint inhibitor nivolumab for treatment of stage 4 nasal malignant melanoma. On examination, his best-corrected visual acuity was noted as 20/20 in the right eye and 20/16 in the left eye. Intraocular pressure was 10mm Hg in both eyes. There were no cells or flare noted in anterior chamber or vitreous, which could indicate Vogt-Koyanagi-Harada disease. Fundus examination showed bilateral macular vitelliform lesions with associated serous retinal detachments. Of note was diffuse thickening of the photoreceptor outer segments and choroid. Fluorescein angiography and indocyanine green angiography did not reveal pooling or leakage, which could indicate metastatic choroidal melanoma.
Diagnosis and management. Bilateral multifocal serous retinal detachment in the setting of diffuse thickening of photoreceptor outer segments and choroid, without indication of ocular inflammation, was diagnosed as iatrogenic impairment of RPE from treatment with nivolumab. The patient maintained a visual acuity of 20/20 in both eyes during his last eye examination visit, which was two months before his systemic condition deteriorated and the patient passed away. The authors noted that in the hypothetical scenario of decreased vision, they would have consulted the patient’s oncologist to discuss altering his systemic medication and starting treatment with topical dexamethasone at the same time.
Discussion. It is believed that immune checkpoint inhibitors, like nivolumab, may promote T-cell directed impairment of RPE cells, which affects RPE cells’ pumping and phagocytosis function, leading to serous retinal detachment and diffuse thickening of photoreceptor outer segments. Immunomodulators, used for management of various rheumatologic disorders and cancer, may cause serous retinal detachment secondary to immune dysregulation. A thorough history, entrance testing, slit lamp exam and a dilated fundus examination supplemented with fundus photographs, macular OCT and visual field test is usually sufficient in careful monitoring of patients to diagnose immune dysregulation related ocular changes. In the event that structural and/or functional changes are noted, communicating this urgently with the patient’s oncologist, rheumatologist and family doctor is essential so the risk versus benefit of altering medication can be thoroughly investigated.
Takeaways
Systemic drugs are commonly associated with various ocular side effects. For example, there are agents known to cause toxic optic neuropathy, which selectively affects the papillomacular bundle and therefore presents as bilateral symmetric painless decrease in vision and color vision along with central or cecocentral visual field defect.2 In addition to toxic optic neuropathy, systemic drugs may cause maculopathies, and in some cases, like the atypical HCQ-related retinal toxicity case discussed above, may lead to changes in the macula region nasal to the fovea and mimic the structural changes seen in toxic optic neuropathy or chiasmal disorders.3 Therefore, as eyecare physicians, we must keep drug-induced ocular side effects, such as maculopathy and optic neuropathy, in our differential in the event of visual, functional and/or structural changes noted in our patients’ eyes.
1. Samarakoon N, Harrisberg B, Ell J. Ciprofloxacin‐induced toxic optic neuropathy. Clin Exp Ophthalmol. 2007;35(1):102-4. 2. Van Stavern GP. Metabolic, hereditary, traumatic and neoplastic optic neuropathies. Continuum (Minneap Minn). 2014;20(4 Neuro-ophthalmology):877-906. 3. Lee JM, Kwon HY, Ahn SJ. Atypical presentations of hydroxychloroquine retinopathy: a case series study. J Clin Med. 2024;13(12):3411.. 4. Wilson ME, Sridhar J, Garg SJ, Forman AR. Spectral-domain optical coherence tomographic imaging of pigmented retinal pigment epithelial deposits in a patient with prolonged minocycline use. JAMA Ophthalmol. 2015;133(11):1360-2. 5. Miyamoto R, Nakashizuka H, Tanaka K, et al. Bilateral multiple serous retinal detachments after treatment with nivolumab: a case report. BMC Ophthalmol. 2020;20(1):221. 6. Bhatti MT. 2020-2021 Basic and Clinical Science Course (BCSC), Section 05: Neuro-Ophthalmology. American Academy of Ophthalmology. 2020. 7. Mititelu M, Wong BJ, Brenner M, et al. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol. 2013 Sep;131(9):1187-97. 8. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015;122(1):110-6. 9. Fraunfelder FT, Fraunfelder FW, Jensvold-Vetsch B. Drug-induced ocular side effects. 8th ed. London; New York: Elsevier; 2021. 10. Patel M, Wingert AM, Sridhar J. Blue sclera and retinal hyperpigmentation in a patient with long-term minocycline use. JAMA Ophthalmol. 2022;140(6):e221848. 11. Yu MD, Bommakanti N, Yonekawa Y, Pulido JS. Minocycline-induced retinal pigment epithelium hyperpigmentation masquerading as age-related macular degeneration: case presentation and proposed mechanism. Am J Ophthalmol Case Rep. 2024;36:102154. |
