If your interests lie in macular and vitreoretinal disease, then this year’s ARVO meeting surely did not disappoint. For the first time, a full-day retina subspecialty symposium was held just before ARVO—giving retinal specialists and general eye care practitioners an additional opportunity to hear about the latest and greatest in retina from a roster of world-renowned experts.
It was the perfect way to gear up for the many sessions at ARVO that reviewed the constantly evolving landscape of treatments and imaging technology and introduced new avenues of retinal research. Attendees were eager to hear the long-awaited results of the Age-Related Eye Disease Study 2 (See “
The Latest on AREDS2 at ARVO 2013."). Other hot topics this year included the role of Eylea in age-related macular degeneration (AMD), the genetics of AMD and several novel treatment approaches to AMD. Presentations also highlighted a new retinal prosthesis that may offer help to patients with retinitis pigmentosa (RP), as well as new ways to treat macula edema.
Retinal Prosthesis
It was a milestone moment for retinal technology in February when the FDA approved the first implanted device to treat adults with advanced RP. The Argus II Electronic Retinal Prosthesis System (Second Sight Medical Products)—which has been cleared in Europe since 2011—should be commercially available in the US later this year. For now, it’s been granted “humanitarian use,” an approval pathway limited to devices that treat or diagnose fewer than 4,000 people in the US each year.

The Argus II Implant (left) attaches to the retinal surface with a tack. The cable that both powers the chip and conducts the image signal from the episcleral housing is seen temporally. An early frame of a fluorescein angiogram (right) in a patient with the Argus II Implant demonstrates some persistent macular perfusion. Images: Elaine Leibenbaum, Julia Haller, MD, and Carl Regillo, MD.
A few studies evaluated this prosthesis, hopefully paving the way for its more widespread use. One study looked at the safety profile of 16 patients in Europe who had received the implant.1040/C0017 The patients were followed on average for 6.2 months, and reported no surgical or serious, device-related adverse effects. Ten patients experienced no surgery or device-related adverse events at all, whereas the other six reported minor adverse effects, such as IOP elevation, nausea, fainting, conjunctival irritation and a retinal tear.
A second study found that the Argus II implant has good long-term reliability, with only one failure in 30 subjects (each with an average of 4.2 years of use, representing more than 125 cumulative patient years).1037/C0014 Further, accelerated lifetime testing demonstrated that finished implants have more than a 10-year lifetime in accelerated testing. Another study confirmed these results, echoing previous tests that demonstrated the ability of the prosthesis to provide visual function over several years.349
Diabetic Retinopathy
Researchers evaluated 759 patients from the RISE/RIDE Phase III trials to see if Lucentis (intravitreal ranibizumab, Genentech) had an effect on the severity of a patient’s diabetic retinopathy.4028 Results showed that a greater proportion of patients in the ranibizumab arm had a two- or three-step regression of diabetic retinopathy on the ETDRS scale vs. those in the sham group. A three-step improvement was achieved at 36 months in 3.3% of the sham group, compared to 15.0% and 13.2% in the 0.3mg and 0.5mg treated eyes, respectively. Over the course of 36 months, 33.9% of the sham-treated eyes developed proliferative diabetic retinopathy, as opposed to only 12.8% and 15.1% of the ranibizumab-treated eyes.
Another study evaluated the safety and efficacy of Macugen (intravitreal pegaptanib, OSI Pharmaceuticals Inc.) combined with panretinal photocoagulation (PRP) vs. PRP alone in the regression of retinal neovascularization in eyes with high-risk proliferative disease.2439/C0140 At six months, the combination of pegaptanib with PRP showed better preservation of best-corrected vision, greater decrease in retinal thickness and maintained visual field better than PRP alone, but showed no major difference in neovascular regression.
A second study of 30 patients compared combination therapy of ranibizumab with PRP vs. PRP alone in treatment-naïve proliferative diabetic retinopathy (PDR).5761/D0008 This study uncovered a greater change in best-corrected vision, a larger decrease in central retinal thickness and a lower incidence of vitreous hemorrhage in the combination treated group––again suggesting that anti-VEGF agents in conjunction with PRP may be preferred to PRP alone.
Additionally, a retrospective study of 78 patients seemed to indicate that metformin may reduce the rate of PDR in type 2 diabetes patients.2249/C0150 In the non-metformin group, 15 patients (45.5%) developed PDR as compared to just 12 patients (27.3%) in the metformin-treated group, indicating a trend of less PDR in the metformin-treated group. A larger study is recommended.
Macular Edema
Several studies are investigating alternative approaches to treat macular edema––either secondary to diabetes or vein occlusion. The MOZART study evaluated the safety and efficacy of an intravitreal dexamethasone implant (Ozurdex, Allergan) in 59 patients with visual impairment from diabetic macula edema (DME).2387/C0088 Investigators noted that, over the six months, central retinal thickness was reduced and acuity improved—28% of patients had 20/40 or better acuity vs. just 6% at baseline. They observed IOP greater than 25mm in 7% of patients, with 4% of patients developing cataracts. No endophthalmitis was reported.
A second study showed positive results using a different intravitreal dexamethasone implant injection (DEX-I), with a gain of more than 10 letters in 27% of cases at two months and 24% at four months.2382/C0084 However, this study showed that recurrence of edema was observed in 76% of cases at four months, leading to re-treatment in more than one-third of cases.
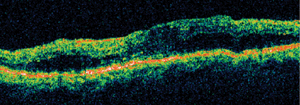
Diabetic macular edema, as confirmed by optical coherence tomography.
Another study evaluated Ozurdex in patients with macular edema from vein occlusions. Forty eyes were treated with Ozurdex and were followed for six to 24 months.254/D0099 Overall, 94% showed initial regression on OCT, lasting an average of 4.2 months, with two lines of improvement. Overall, 59% improved 14.2 letters on average, while 10% worsened and 31% remained the same. Approximately 19% had elevated IOP and were treated with drops. In eyes that were not previously treated, the results were even better—86% showed improvement. Half of the patients required retreatment, with an average of 1.6 treatments per year. The results seem to indicate that Ozurdex may be an effective treatment in such patients––even those who did not respond well to anti-VEGF agents.
Other research looked at the role of laser, as well as anti-VEGF in combination with laser, in the treatment of macular edema. The LLOMD study evaluated 15 eyes of 13 patients with reduced visual acuity secondary to diabetic macular edema who had a mean VA of 20/100.2396/C0097 At six months, the mean VA gain was 12.6 ETDRS letters, with the central retinal thickness decreasing an average of 76.7µm in patients who received laser in combination with ranibizumab. Additionally, 37.5% of patients required a second injection at six months. However, with the addition of laser, the study showed that the number of injections needed over the first year was greatly reduced compared to previous studies of injections alone. In total, the researchers determined that approximately 10 injections are needed during the first year. Further, they concluded that adding macula grid laser to ranibizumab injection may reduce the economic burden of treatment.
Two additional studies revealed that reduced-energy focal macular photocoagulation could have advantages over traditional focal macular laser. 2375/C0076,2416/C0117 Both seemed to indicate that, by reducing the laser exposure when performing the procedure, there were decreases in CRT and increases in vision––with potentially less collateral damage and inflammation to surrounding viable tissue. More research is needed to investigate whether reduced-energy focal macular photocoagulation could replace more traditional laser therapy as the standard.
Eye on Eylea
Several reports evaluated Eylea (aflibercept, Regeneron Pharmaceuticals), the latest FDA-approved anti-VEGF agent for the treatment of wet AMD. A number of these looked at the role of Eylea in patients whose choroidal neovascularization did not respond to other agents, namely Lucentis (ranibizumab, Genentech) and Avastin (bevacizumab, Genentech/Roche).
One study evaluated 41 eyes of 34 such patients—77% of these patients had a good response to Eylea after one month, demonstrating decrease in central retinal thickness and absorption of subretinal fluid.4176/A0094 Best-corrected visual acuity improved in these patients to 20/74, from 20/122.5 at baseline.
A second study evaluated 60 eyes of 52 patients that did not respond after five consecutive injections of the other agents.3806/B0116 After three Eylea injections, 28 eyes (46.7%) displayed improved acuity, while 18 eyes (30%) showed decreased acuity, and 14 (23.3%) had no change in acuity at three months.
Lastly, a study evaluated 19 eyes of 17 patients receiving Eylea as primary therapy, with dosing as needed.3817/B0127 Over a 20-week period, patients received on average 1.84 injections, with an interval between injections of approximately 11 weeks. Five of these patients were determined to be non-responders to other anti-VEGF agents. Of these five, four responded positively to Eylea, indicating again that Eylea may be an effective alternative for patients who do not respond to other agents. Also, this study seems to indicate that the interval to repeat injections may be longer with Eylea than the other agents.
However, a separate study looked at the costs associated with Eylea.3838/B0148 The researchers hypothesized that, despite fewer injections, the cost of treatment per patient would actually increase. The study reviewed the records of 30 patients treated for wet AMD from 2011 to 2012 at the Cincinnati Eye Institute. The average duration between Avastin or Lucentis injections was 29 days, as opposed to 34 days with Eylea injections. No complications were noted in any groups. Total cost over the six months was $3,700 for Avastin, $96,000 for Lucentis and $366,300 for Eylea. This study suggests that while Eylea may reduce the frequency of injections, office visits and possibly complications, it appears to add considerable health care costs per patient.
New AMD Treatments
Several studies evaluated novel treatments for AMD. One study investigated the safety and feasibility of an episcleral brachytherapy device (SMD-1) for wet AMD.3787/B0097 Six patients received radiation for five and a half minutes to the macular CNV using a brachytherapy probe adjacent to the macular sclera via a subtenon retrobulbar approach. Patients also received concomitant anti-VEGF injections, as needed. The procedure was readily performed and well tolerated, with no adverse effects. At three months, all patients experienced improved best-corrected vision, with a mean gain of 19 ETDRS letters. At 12 months, three patients continued to demonstrate improved vision of seven letters on average, and two of those patients did not require any additional injections. All patients had reduced macular thickness compared to baseline, but two patients did demonstrate a reduction in vision.
Another study evaluated the safety of 1% CLT-005 topical eye drops, designed to inhibit Stat3, which has been associated with neovascular and inflammatory processes in animal studies.1716 The researchers determined that the drug was able to deliver the active ingredient to the RPE/choroid in animal eyes, without adverse effects—paving the way for additional studies regarding its role in the treatment of AMD or geographic atrophy (GA).
Australian researchers looked at the progression of early AMD after treatment with nanosecond pulse laser compared to patients with a natural history of AMD.4146/A0064 They treated 48 patients with bilateral high-risk AMD with ultra-low energy laser in 12 spots around the macula of one eye. At 12 months, three of the 48 treated participants progressed to GA, while seven of the 70 control group progressed. At 24 months, four in the treated group and nine in the control groups progressed to GA, suggesting that a single course of nanosecond laser intervention may potentially reduce the odds of progression to advanced AMD. A larger randomized controlled study is now underway.
Another study evaluated the safety and tolerability of an extrafoveal subretinal injection called rAAV.sFlt-1, an anti-VEGF gene therapy for AMD, in elderly patients.4504 Twelve patients underwent the procedure with minor adverse effects and no evidence of local or systemic toxicity. The researchers noted that this injection should be further evaluated as a potential strategy for long-term anti-VEGF therapy.
Research continues on Emixustat HCL, a novel orally administered agent in development for the treatment of GA associated with dry AMD.4506 Emixustat HCL is a rod visual cycle modulator that inhibits isomerase activity and reduces retinal toxins, such as A2E, which damages the RPE and overlying photoreceptors. Four dose levels and two dose regimens were examined in 72 patients who were followed for 90 days. No adverse systemic effects of concern were noted, with just two patients experiencing treatment-related events. All ocular adverse effects resolved upon drug cessation, and were mild with no severe events observed. Results were encouraging, and a long-term Phase II study in now underway to evaluate its role in GA patients.
Other studies looked at using existing therapy more effectively. A team of researchers in Italy evaluated whether ketorolac eye drops combined with ranibizumab intravitreal injections would provide additional efficacy over ranibizumab alone in wet AMD.4175/A0093 Sixty eyes were divided into two groups: one received ranibizumab alone, and one was treated with ranibizumab plus ketorolac BID for six months. At the end of six months, there was no statistically significant difference in best-corrected vision or number of injections required. However, the mean six-month change in central macular thickness was 146.53µm in the combination group, while the change was 106.88µm in the ranibizumab-only group. This is the first study to identify an additional effect of ketorolac eye drops combined with ranibizumab. More studies would be needed before a change in current protocol would be appropriate.
Two separate studies evaluated photodynamic therapy in combination with anti-VEGF injections.4509,3790/B0100 Both indicated that this therapeutic combination might be an effective way of improving acuity in patients with wet AMD, while perhaps reducing the overall number of treatments needed. In one of the studies, 96.2% of eyes lost fewer than 15 letters, and 27.3% gained 15 or more letters.
Genetics in AMD
Genetics in eye care have been garnering a lot of attention lately, specifically the role of genetics in AMD.
One study evaluated data from the 100 Genomes Project to confirm the contribution of known genetic risk factors for AMD.6166/C0051 This investigation revealed that, in the population of European descent, CFH has the largest attributable risk (25.6%), followed by ARMS 2 (22.5%), then C3 (9.1%) and CST3 (5.8%). In other populations, the risk allele in ARMS2 is the major contributor to risk, followed by CFH. In Asian and African populations, CST3 takes precedent over C3 as the third strongest contributor to AMD risk.
In Spanish patients, a study found that CFH and CB genes, combined with environmental risk factors such as smoking and body mass index, were associated with an increased risk of GA.6183/C0068 A second abstract confirmed the role of CFH gene in AMD risk in a cohort of Brazilian AMD patients.6175/C0060
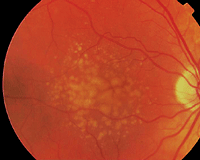
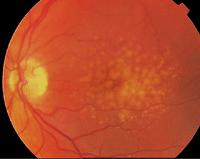
This patient is at high risk for AMD due to multiple confluent drusen in both eyes. Perhaps genetic testing could one day identify patients like this earlier.
In another study evaluating the genetic contribution of AMD in 38 Armenian patients, researchers found no genetic differences in the risk alleles compared to a Caucasian population.6196/C0081 All of this research indicates the genetic factors that could influence the development or AMD may be very similar across different groups.
Interestingly, some of these same studies seem to suggest that the HDL-related CETP gene may be associated with AMD in African Americans, pointing to a potential risk modifier in lipid pathways.6168/C0053
An abstract submitted by Johanna Seddon, MD, ScM, identified three new genes that may add to the predictive power of risk models for progression to advanced AMD.6178/C0063 They are the R1210c mutation in CFH, and variants to the genes COL8A1 and RAD51B. She suggested that these new genes will be useful for AMD surveillance in the future, along with genes that have already been identified and established factors such as drusen size, baseline AMD status, demographics and environmental factors (including smoking, age and body mass index).
Additional studies attempted to see if there was a link between genetic profile and response to treatment. One study evaluated the genetic profile of 835 patients from the CATT (Comparison of AMD Treatment Trial) trial to determine if certain genotypes responded better to treatment than others.6187/C0072 Results revealed there were no strong associations between the studied genotypes and response to anti-VEGF treatment.
A second study evaluated the IVAN study and also was unable to find any associations between genetic profiles and response to anti-VEGF treatment.6185/C0070 However, another study of 43 patients seemed to indicate that patients with high-risk alleles for AMD responded more poorly to treatments than those with low-risk alleles.6186/C0071
This link of genetic profiles to treatment response may continue to be investigated, as this could bring us closer to personalized treatment of AMD––based on genetic factors and other components.
Conducted at 82
clinical sites across the US from 2006 to 2012, the trial included 4,203
participants, ages 50 to 85. The AREDS2 subjects consented to either
take the original AREDS formulation or a randomly assigned variation of
the AREDS formulation. The main
outcome measurement was progression to advanced AMD, neovascular or
central geographic atrophy. Progression to cataract surgery and
progression of lens opacity was a secondary outcome.The addition of
lutein and zeaxanthin, DHA and EPA, or both to the AREDS formulation in
primary analysis did not further reduce the risk of progression to
advanced AMD. However, because of increased incidence of lung cancer in
former smokers, lutein with zeaxanthin may be an appropriate carotenoid
substitute of beta-carotene in the original AREDS formulation.
The
comparison of low-dose vs. high-dose zinc showed no evidence of a
statistically significant effect, so a clinical recommendation cannot be
reached. Lastly, daily supplementation with lutein/zeaxanthin had no
statistically significant overall effect on rates of cataract surgery or
vision loss. It will take some time to digest these results and see how
they should be implemented in practice.
The Latest on AREDS2 at ARVO 2013
The Age-Related Eye Disease Study 2
(AREDS2) Research Team presented the results of the multi-center
randomized, controlled clinical trial of oral supplementation with
lutein/zeaxanthin (10mg/2mg) and/or omega-3 long-chain polyunsaturated
fatty acids (1,000mg) for the treatment of AMD and cataract at the ARVO
meeting.

