 |
A 61-year-old woman presented for follow-up of primary open-angle glaucoma (POAG) with an ongoing report of feeling grittiness and tearing in both eyes. She has a history of bilateral trabeculectomy with mitomycin C with clinical failure in the left eye. She takes Vyzulta (latanoprostene bunod 0.024%, Bausch + Lomb), Rhopressa (netarsudil 0.02%, Alcon) and dorzolamide-timolol in the left eye only with on-label dosing and reported excellent adherence. Additional procedures for the left eye were not planned by the operating physician leading to release from care. Systemically, she has seropositive rheumatoid arthritis, for which she takes 200mg of hydroxychloroquine BID (for the past four years) in addition to weekly etanercept (Enbrel, Amgen) subcutaneous injection. Best-corrected visual acuity was 20/25 OD and OS.
Her SPEED score was 21/28, and she was using nonpreserved tear substitutes two to four times daily in addition to a moist heat mask and eyelid hygiene products. She had significant conjunctival injection, coalesced inferior staining bilaterally, reduced tear meniscus and inspissated meibomian glands which were poorly expressible. Intraocular pressure (IOP) was 5mm Hg in the right eye with a formed anterior chamber and 11mm Hg in the left eye with central corneal thicknesses of 479µm OD and 467µm OS. She had advanced glaucomatous optic neuropathy bilaterally and corresponding constricted visual fields. There was no evidence of early ellipsoid zone loss on SD-OCT.
When managing multiple chronic ocular conditions, dry eye disease often takes a secondary or tertiary seat, despite having a significant impact on quality of life and a important impact on adherence to IOP-lowering therapy.1,2 For someone in such a case, treatment considerations are nuanced and may require multiple strategies, including prescription medications and in-office procedures.
 |
|
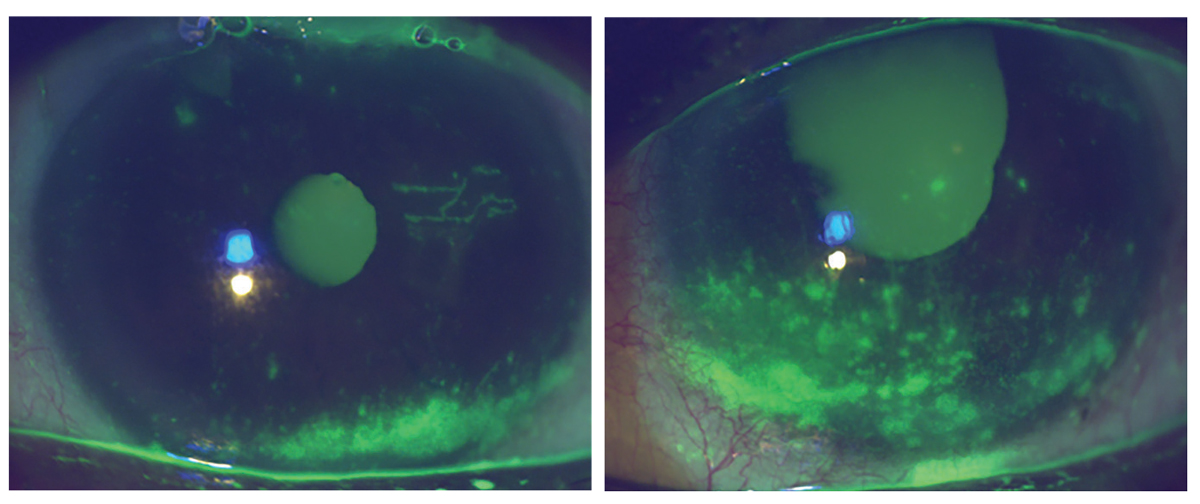
Right and left anterior segment photographs demonstrating corneal epithelial staining, reduced tear meniscus and conjunctival injection. Click image to enlarge. |
The Basis for Treatment
In dry eye disease, the positive feedback loop that precipitates inflammation and ocular surface damage has formed the basis of therapeutic strategies that disrupt the cycle by targeting evaporation, inflammation or tear production to restore tear film homeostasis.1,3 Risk factors for the development and exacerbation of the condition require attention to systemic diagnoses, systemic medications, concomitant ocular conditions and their treatment strategies, history of ocular surgery, in addition to environmental and lifestyle features.1,4-6
Glaucoma and Dry Eye
Individuals with glaucoma are more likely to develop dry eye disease than the general population, with risk factors driven by glaucoma severity, duration of treatment, benzalkonium chloride (BAK)-containing therapies, history of filtration surgery and number of therapies.6-9 These topical IOP-lowering agents may be the low-hanging fruit for symptoms and signs related to dry eye and are often contributory. Removal or reduction of BAK-containing medications may not be possible, nor will removal alone cure the underlying multifactorial disease state.
The LiGHT study provided long-term evidence that SLT is a safe and effective therapy for newly diagnosed patients with POAG or ocular hypertension.10 Interestingly, an American health claims-based report determined that approximately 44% of individuals diagnosed with OAG require a modification to their initial treatment within the first four years of therapy, with nearly 30% of those patients requiring a second modification in the same time period.11 Incidence of filtration surgery increases with time following diagnosis, from 3.1% at five years to 5.4% at 10 years.12 Cumulative risk of blindness in one eye increases with time to 13.5% at 20 years following diagnosis. Managing patients through their lifetime often requires multiple escalations in therapy.13,14
Trabeculectomy with antimetabolite use has been demonstrated to increase risk of symptomatic dry eye.15 Conjunctival irregularities due to the presence of a filtration bleb alter tear film distribution and stability and reduce tear break-up time.9 Antimetabolites such as mitomycin C (MMC) and 5-fluorouracil (5-FU), when used intra- and postoperatively, have led to increased surgical success; however, they may lead to meibomian gland loss and limbal stem cell deficiency.9 Increased inflammation and hyperosmolarity can stimulate remodeling of the bleb wall, which may drive fibrosis and reduce functionality.9
Systemic medications and systemic inflammatory conditions that impact the lacrimal functional unit can result in an unstable tear film and lead to ocular surface damage, so be sure to identify those in the medical history.4-6 Also, note systemic inflammatory conditions, including autoimmune thyroid disease, rheumatoid arthritis and Sjögren’s syndrome, as well as neurological conditions, including trigeminal neuralgia, Parkinson’s disease and chronic viral infections.1 In particular, rheumatoid arthritis has been associated with hyperosmolarity, a central etiology of dry eye disease.3,16
 |
|
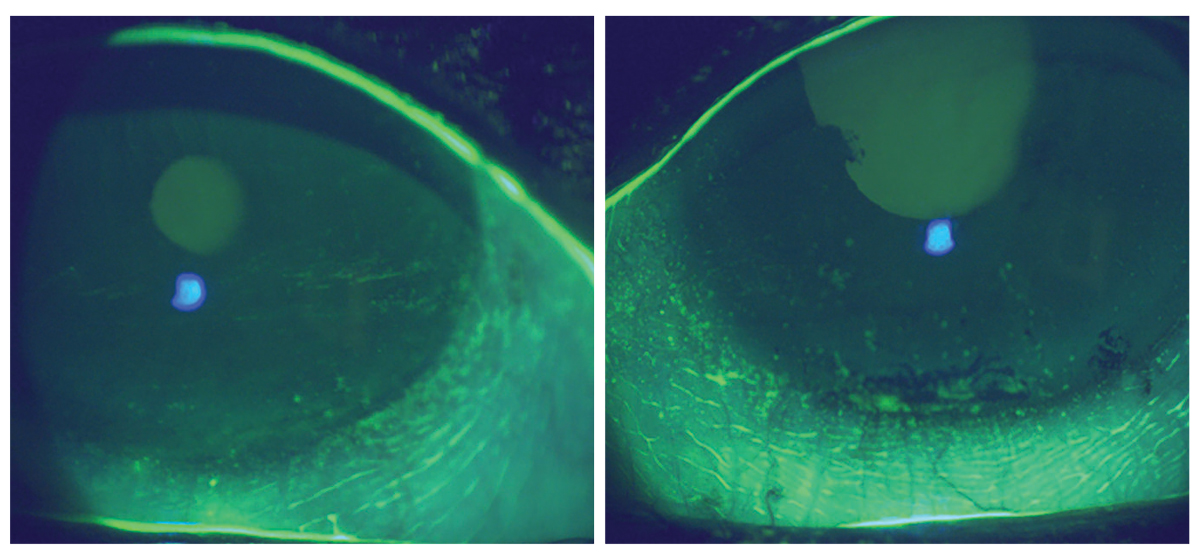
Eight weeks following initiation of prescription medication therapy with continued use of artificial tears, eyelid hygiene and moist heat mask use. Click image to enlarge. |
Individualized Treatment
Current prescription options have provided the ability for a truly individualized approach that allows for direct targeting of underlying mechanisms, including evaporation, inflammation and tear production, either alone or in combination. Despite the patient’s significant IOP-lowering medication and BAK load and taking note of the unilateral use and bilateral but asymmetric clinical presentation, her topical regimen did not seem to be a central factor in her dry eye disease. Still, caution was taken to ensure she was not overtreated. She reported a history of steroid response and was highly reluctant to a short course of topical steroids. Her travel schedule also limited her ability to return for evaluation within an appropriate time course, leading to deferral of a topical steroid despite its role in reducing ocular surface inflammation. She had felt that she was at her maximum threshold for topical medication instillation use, despite her candidacy for a topical ophthalmic immunomodulating agent or topical agent to reduce tear film evaporation.
Considering the patient’s preferences, she was prescribed Tyrvaya (varenicline nasal spray 0.03mg, Viatris), in addition to current nonpreserved tear substitutes, eyelid hygiene and a moist heat mask. The expected efficacy and tolerability profile of the nasal spray were discussed, including the expected adverse effect of sneezing following instillation, and the patient demonstrated correct placement of the bottle tip in the examination room.
Eight weeks later, she returned for follow-up and reported significant improvement in symptoms and improvement in vision. Her SPEED score improved to 10/28, and while mild epithelial staining was present, it and the conjunctival injection significantly improved bilaterally. An update was provided to her rheumatologist regarding ongoing therapy with a query of investigation into Sjögren’s syndrome.
Despite the patient’s success with her current prescription therapy, she still displays corneal staining and has moderate symptoms of dry eye disease. She represents an ideal candidate for in-office heat- or light-based treatment related to her meibomian gland dysfunction, with the understanding of likelihood of flares and necessity of ongoing adjustments to dry eye treatment, along with continued treatment, POAG monitoring and evaluation of risk for developing hydroxychloroquine toxicity.
Dr. Steen is an associate professor at Nova Southeastern University College of Optometry where she serves as director of the Glaucoma Service, coordinator of the Primary Care with Emphasis in Ocular Disease Residency and teaches courses in glaucoma and ocular pharmacology. Her financial disclosures include Bausch & Lomb, Santen, Ocuphire and Carl Zeiss Meditec.
1. Amescua G, Ahmad S, Cheung AY, et al.; American Academy of Ophthalmology Preferred Practice Pattern Cornea/External Disease Panel. Dry eye syndrome Preferred Practice Pattern. Ophthalmology. 2024;131(4):1-49. 2. Nordmann JP, Auzanneau N, Ricard S, et al. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003;1:75. 3. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. 4. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-65. 5. Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II sex, gender and hormones report. Ocul Surf. 2017;15(3):284-333. 6. Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-38. 7. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618-21. 8. Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2012;11:0. 9. Agnifili L, Figus M, Sacchi M, et al. Managing the ocular surface after glaucoma filtration surgery: an orphan topic. Graefes Arch Clin Exp Ophthalmol. 2024;262(7):2039-56. 10. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial: six-year results of primary selective laser trabeculoplasty vs. eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology. 2023;130(2):139-51. 11. Schwartz GF, Patel A, Naik R, et al. Characteristics and treatment patterns of newly diagnosed open-angle glaucoma patients in the United States: an administrative database analysis. Ophthalmol Glaucoma. 2021;4(2):117-25. 12. Virtanen A, Haukka J, Loukovaara S, et al. Incidence of glaucoma filtration surgery from disease onset of open-angle glaucoma. Acta Ophthalmol. 2024;102(2):192-200. 13. E Gramer, G Gramer. Age of the patients at the time of diagnosis in different glaucomas: a prospective study. Invest Ophthalmol Vis Sci. 2002;43:3422. 14. Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. 2013;156(4):724-30. 15. Lam J, Wong TT, Tong L. Ocular surface disease in posttrabeculectomy/mitomycin C patients. Clin Ophthalmol. 2015;9:187-91. 16. Schargus M, Wolf F, Tony HP, et al. Correlation between tear film osmolarity, dry eye disease and rheumatoid arthritis. Cornea. 2014;33(12):1257-61. |

