24th Annual Glaucoma ReportFollow the links below to read other articles from annual update on glaucoma: MIGS Madness: An Atlas of Options Comanaging Invasive Glaucoma Surgeries Glaucoma: Lifestyles of the Antioxidant Rich and Famous (Earn 2 CE Credits) |
Experts have yet to reach consensus on a universally accepted etiology of glaucoma. However, they do agree on this: lowering intraocular pressure (IOP) is the only modifiable risk factor for slowing its progression. Topical pharmacotherapy is the traditional first-line approach, and the options are more abundant than ever.
This article, part three of our Take Charge of Glaucoma Series, explores the many meds ODs can employ, with advice on when to use which therapy for which patients.
Early Autonomics
Medications that act on the autonomic nervous system (i.e., cholinergics and adrenergics) have been a basis of glaucoma therapy since the 1800s.1,2 Cholinergics induce miosis, which stretches and stimulates the trabecular meshwork (TM) to increase aqueous outflow there and to Schlemm’s canal.3 Members of this class include pilocarpine, carbachol, physostigmine, neostigmine and ecothiophate. Of those, only pilo is still in routine use. Treatment exhibits a dose-related response with a decrease in IOP of about 20% when prescribed QID.4
Take Charge of GlaucomaFollow the links below to read other articles from our four-part glaucoma series: |
Though effective, cholinergic use is limited by its ocular and systemic side effects. These include ciliary muscle spasm (with associated headache and induced myopia), miosis, corneal toxicity, redness, uveitis, possible cataract formation, respiratory depression and gastrointestinal distress. Further, newer medications have greater IOP-lowering efficacy, which leaves cholinergics reserved for specific cases where the miotic effect may have an added benefit, such as acute angle closure.
Adrenergic agonists, on the other hand, impact the alpha or beta adrenergic receptors (or both if non-selective). Epinephrine, the primary non-selective agent, reduces IOP by first decreasing aqueous production and then increasing outflow through the TM.5Unfortunately, it has limited application due to significant systemic side effects. Dipivefrin, a prodrug of epinephrine developed in the 1970s, allows use of much lower concentrations of the parent compound, with fewer systemic effects.6 Nonselective adrenergics see minimal use today except in cases where other drugs may be contraindicated.
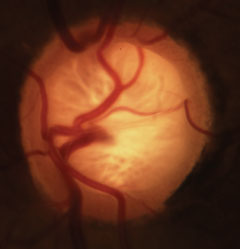 |
| Glaucomatous cupping with characteristic loss of neuroretinal rim, lamina and alteration of the vasculature. Click image to enlarge. |
Beta Blockers
Adrenergic antagonists, or beta (β)-blockers, inhibit aqueous production and represent the standard by which new medications are compared. Specifically, β-blockers reduce ultrafiltration, which limits the availability of aqueous humor substrate available for transmission into the posterior chamber.1,7,8 These medications can be nonselective, meaning they inhibit both isoforms of the β-adrenergic receptors (β1 and β2), or cardioselective, which have much greater affinity for the β1 receptor. β2 is the eye’s predominant adreno-receptor, so nonselective agents will have a greater impact on IOP control.9,10
Topical β-blockers include timolol, levobunolol, metipranolol, carteolol and betaxolol. Only betaxolol is cardioselective—which makes it helpful in certain contraindications, but it may be less effective at reducing IOP.11β-blockers reduce IOP by 20% to 30% and may be dosed twice daily. They can also be dosed once daily, particularly when using gel-forming solutions due to their increased ocular contact time.12,13 Adrenergic antagonists may have less impact during sleep, so care should be taken when β-blockers are dosed close to bedtime, especially if prescribed as a once daily regimen.14,15 If patients are taking systemic β-blockers, the ocular hypotensive effect of topical β-blockers is reduced, and other classes of topical medications could be considered.16
Although this class of medication should mostly be avoided in pulmonary or cardiac conditions, in select cases it may be reasonable to consider beta-blocker therapy. However, this should be done with the consent of the appropriate specialist (cardiology or pulmonary).
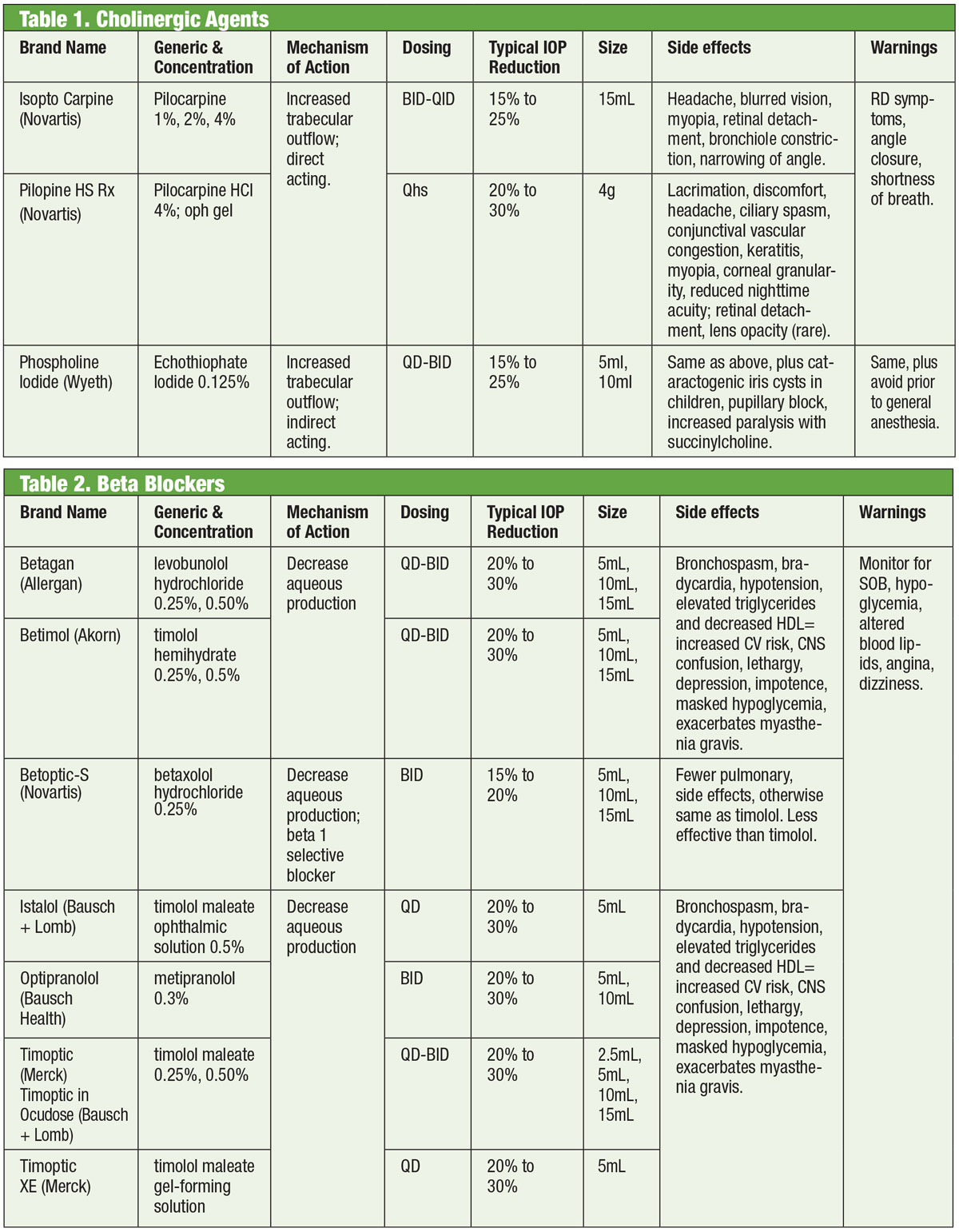 |
| Tables: Bruce Onofrey, OD, RPh. Click image to enlarge. |
Generally, however, β-blocker use should be avoided in those with atrioventricular block, sinus bradycardia, and obstructive pulmonary disease.17
Carbonic Anhydrase Inhibitors
Researchers have acknowledged the ability of oral carbonic anhydrase inhibitors (CAIs) to decrease IOP since the 1950s.18 They accomplish this by suppressing aqueous production.18 However, systemic side effects (such as fatigue, gastrointestinal disturbances and paresthesia) limit their chronic use in glaucoma.18 Oral CAIs are still used in cases when topical CAIs cause hypersensitivity or when the use of drops is precluded, as well as in cases of acute angle closure.18 Attempts to formulate a topical variety succeeded with the introduction of dorzolamide in the mid-1990s and, soon after, brinzolamide.19
There are at least seven different isoenzymes of carbonic anhydrase (CA), with CA-II in the ciliary processes being predominately involved with aqueous production.18 Both dorzolamide and brinzolamide are potent inhibitors of this isoenzyme, but they have several clinically relevant differences. For example, dorzolamide has a 5.6 pH while brinzolamide has a 7.5 pH. Additionally, brinzolamide is available as a suspension. These are just some of the properties that can account for the products’ individual side effects, which can include stinging in the case of dorzolamide and blurred vision in the case of brinzolamide.19
Though the drugs FDA-labeled for TID dosing, some practitioners opt for BID administration. For brinzolamide, phase III trials report clinically equivalent IOP reductions with either BID or TID regimens.20 Others report no statistically significant differences between BID or TID dosing with dorzolamide.18 Alternatively, others advocate TID dosing for monotherapy and BID dosing when used as adjunctive therapy.21
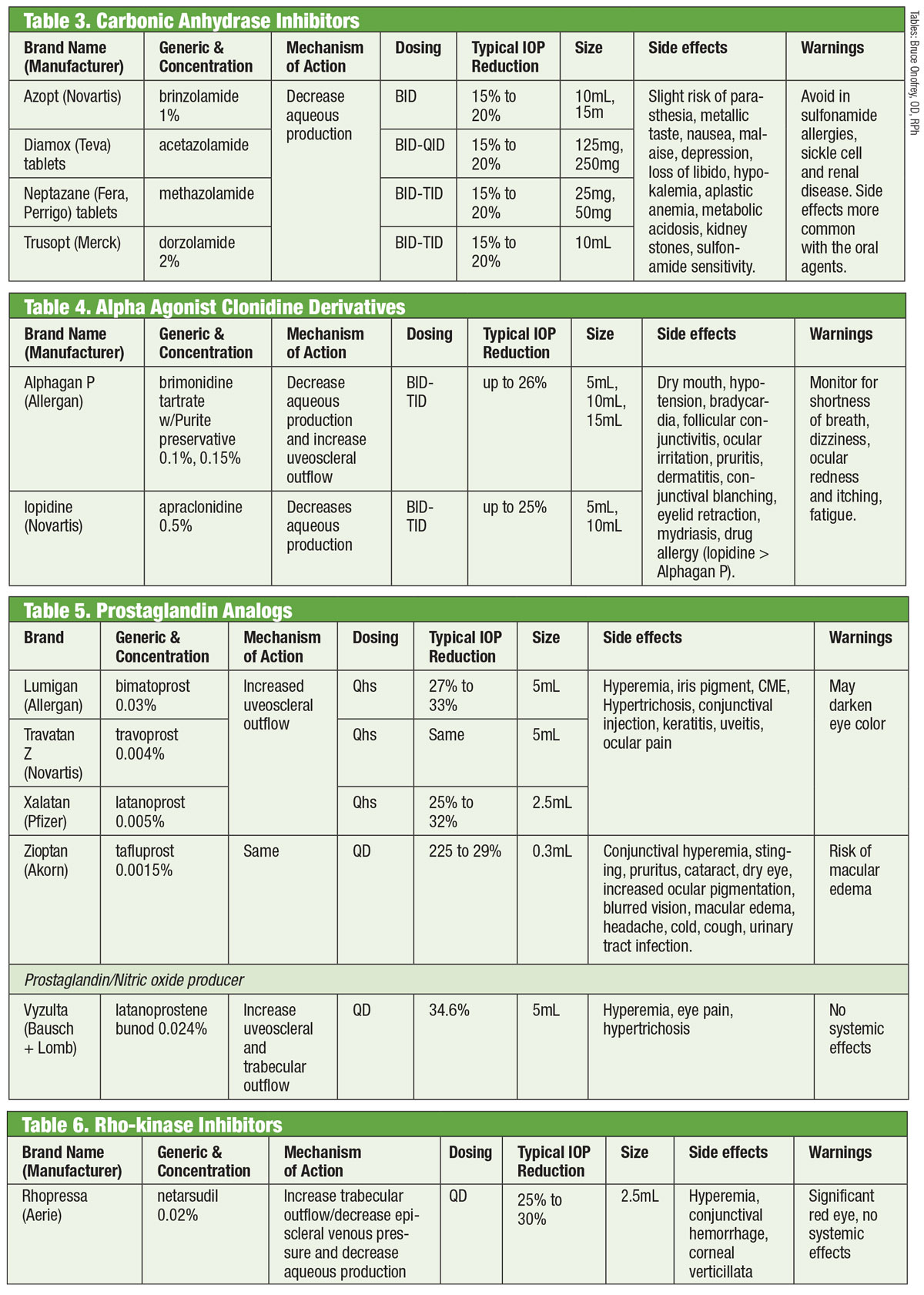 |
| Tables: Bruce Onofrey, OD, RPh. Click image to enlarge. |
Alpha Agonists
Clonidine represented the earliest alpha agonist (AA) effective for lowering IOP. However, even in topical form it produced profound systemic side effects of bradycardia, sedation and hypotension.22 Apraclonidine’s amide group substitution decreased blood-brain barrier penetration, and thus side effects. Unfortunately, its diminished efficacy over time and an increased incidence of ocular allergy limits it to short-term use.
Introduced in 1997, brimonidine is a potent AA with 32 times more selectivity for β2 adrenoreceptors than apraclonidine.22 It lowers IOP through a dual mechanism of decreasing aqueous production and increasing uveoscleral outflow.23 Like its predecessor, it has a notable allergy rate (up to 20%), which may occur up to eight months after initiation of therapy.22 Three formulations have been developed, each with similar efficacy: 0.2% with benzalkonium chloride (BAK); and 0.15% and 0.1% with the preservative Purite.24 Allergan has since discontinued the BAK-preserved formulation.
The Straight DopeMedical marijuana has been making big news of late, thanks to a number of legislative changes in the United States. Although it’s been researched as a method to reduce IOP since the 1970s, investigators have only found it capable of lowering IOP for brief periods of time. That, coupled with its rather hefty side-effect profile, makes it a poor candidate for treatment. However, research says it may have a role to play for “end-stage glaucoma patients who have failed maximal medical therapy and surgery or who are poor surgical candidates.”
|
Though side effects mostly correlate with prostaglandin use, the literature shows brimonidine-induced anterior uveitis.25 Duration ranged from seven days to five years with a mean of nearly 20 months.
Prostaglandin Analogs
These provide the most robust IOP reduction of all drops by enhancing uveoscleral aqueous outflow. Prostaglandin analogs (PGAs) bind to receptors in the ciliary body and induce smooth muscle relaxation, and alter the extracellular matrix within the ciliary muscle to increase aqueous outflow through uveoscleral routes.26 Dosed once per day, commonly at bedtime, PGAs yield a 30% to 35% IOP reduction; options include latanoprost, bimatoprost, travoprost and tafluprost (supplied in a single dose, preservative-free option).26 The most common side effect of PGAs is conjunctival hyperemia, often in the first few weeks after initiation of therapy.26 Ocular irritation, exacerbation of existing inflammatory conditions (macular edema, iritis), atrophy of the periorbital fat pad, pigmentation of periocular skin, eyelashes and iris and hypertrichosis may also be seen.26
A new variant in the prostaglandin analog category is latanoprostene bunod 0.024%. This compound has a dual mechanism: increasing uveoscleral outflow and enhancing trabecular meshwork outflow through the impact of nitric oxide.27 The eye breaks down latanoprostene bunod twice to yield the active components latanoprost acid and nitric oxide.28 The latter impacts a signaling pathway that relaxes contractile components in the TM, which increases outflow.28 The additional impact drops IOP >1mm Hg vs. latanoprost alone across multiple time points, with total IOP reduction ranging from 7.5mm Hg to 9.1mm Hg. Side effects with latanoprostene bunod were comparable to those of latanoprost alone, with possibly less periocular pigmentation and hypertrichosis, according to investigators.28,29
ROCK Inhibitors
In a normal eye, the main drainage pathway for aqueous humor is the TM. Resistance to aqueous humor through this structure is increased in patients with glaucoma, raising IOP.30 Until recently, glaucoma medications failed to target this structure. That changed in 2017 with the introduction of Rhopressa (netarsudil 0.02%, Aerie), a rho-kinase (ROCK) inhibitor.
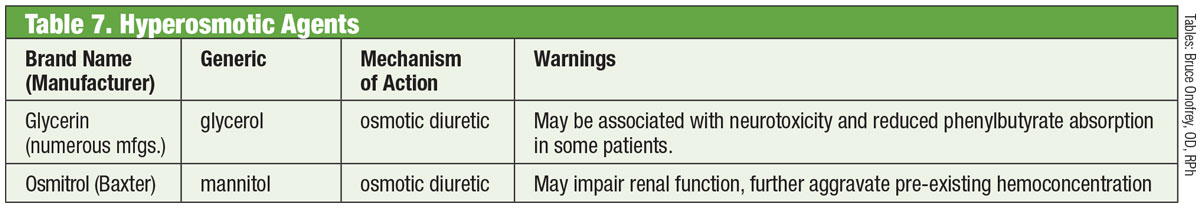 |
| Table: Bruce Onofrey, OD, RPh. Click image to enlarge. |
Rho-kinase is widely expressed in many tissues, including the TM, where it promotes the assembly of actin stress fibers and regulates cell contraction.31 ROCK inhibitors increase aqueous outflow by decreasing actin and myosin-driven cellular contraction and reducing extracellular matrix protein production.32 Rhopressa also has inhibitory action against norepinephrine transporter (NET), making it a ROCK/NET inhibitor. The NET mechanism may be the result of reduced blood flow to the ciliary body through norepinephrine-induced vasoconstriction, which results in decreased aqueous production.33 In addition, netarsudil also decreases episcleral venous pressure (EVP), thus providing multiple avenues for IOP reduction.32 The most common ocular side effect is ocular hyperemia (about half of treated patients), which for the most part is mild, transient and self-resolving.32
Of note, rho itself plays an important role in axon growth and guidance, as well as the regulation of neuronal survival and death.34 Researchers found that, following optic nerve injury, topical application of netarsudil reduces retinal ganglion cell death and promotes axonal regeneration.34
Getting in the MixSometimes patients need more than what off-the-shelf products offer. Patient sensitivity or ocular surface toxicity from chronic use of preservatives may lead to a search for alternatives. Some compounded drugs are available preservative free. If compliance is an issue, these mixtures can contain up to four medications in a single bottle. Further, many fixed combinations have been used effectively outside of the United States, but are not currently approved by the FDA; compounding gives US doctors access to those regimens. If cost is a limitation, compounding could be an added advantage. While the compounded product is often not covered by the patient’s insurance plan, the price of one or two combination products may be more cost effective than that of multiple single-medication options, even generic formulations. Two compounding pharmacies offer multiple pre-set variations: Simple Drops from Imprimis Pharmaceuticals and the Omni prodcut line from Ocular Science. Additional customization is possible from each, too. Although the individual agents are FDA approved, particular combinations may not be. It is up to the practitioner to weigh the risks and benefits of compliance. Compounded Glaucoma Products: |
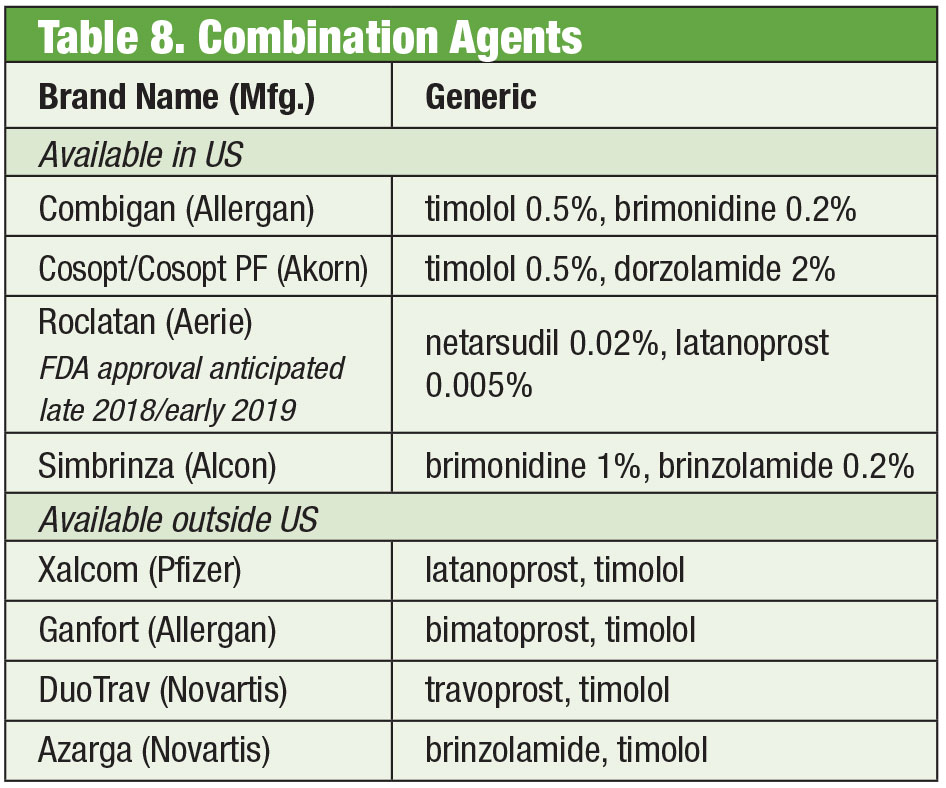
Fixed Combination Products
Despite the efficacy of these individual products, it is common for many glaucoma patients to require multiple therapies to control their condition.35 When dual therapy is necessary it may be best to offer the same dispenser, promoting increased compliance and reducing impact on the ocular surface from toxicity.36 Timolol pairs with brimonidine, (as Combigan, Allergan) and dorzolamide (as Cosopt, Akorn), both dosed twice per day, with a preservative-free option available for the timolol-dorzolamide product. Another twice daily combination—Simbrinza—couples brimonidine and dorzolamide. The fixed combinations show good efficacy both as primary therapy and in addition to PGAs. Side effects are comparable to the individual components.
Adherence/Patient Perception
Although the decision to recommend treatment can be complex and is contingent on many factors, once the doctor and patient agree to initiate therapy, certain background facts must be acknowledged and basic tenets must be employed.
 |
| Click image to enlarge. |
Visual field progression and disease severity is linked to poor adherence.37 Patients with chronic medical conditions use, on average, 30% to 70% of their prescribed medication doses, and 50% discontinue their medications within the first few months of therapy, according to one study.38 Adherence to glaucoma medications is similarly poor to that of other chronic conditions.38 Accordingly, there may exist an efficacy-effectiveness gap where, although it has been proven that topical medications are efficacious in large clinical studies, in practice they can be ineffective due to patient noncompliance.39
Major factors that contribute to poor adherence in glaucoma are, among others; side effect profile of medications, cost of therapy, patient education and the doctor-patient relationship.40 Additionally, the disease may be asymptomatic until late in its course, with lack of awareness of visual field loss.41
And Now For Something Completely DifferentMedications have been the backbone of glaucoma management for well over a century, and development of new drugs continues unabated. But some researchers are looking for alternatives beyond the bottle. Glasses embedded with an electromagnetic coil, coupled with a contact lens containing a trace of gold, may one day help to lower IOP. Developed by a company called Bionode, the combo is designed to generate an electric current that flows through the ciliary muscles to stimulate the natural drainage pathway and decrease IOP.
|
An additional barrier to adherence is difficulty with drop instillation and dosing schedule.42 In fact, up to 80% of patients contaminate their drops by touching their face, up to 61% do not instill exactly one drop and, most critically, up to 37% miss the eye with the drop.43 The practitioner should never assume the patient is proficient with drop instillation. Prior to initiating drop therapy, teach the patient how to appropriately instill drops and have them successfully demonstrate instillation prior to leaving the office. This can be efficiently delegated to an optometric technician and reinforced with an educational handout on technique (printable PDF at www.glaucoma.org/treatment/eyedrop-tips.php) as well as the use of videos such as one produced by the Glaucoma Research Foundation (www.glaucoma.org/treatment/putting-in-eye-drops.php).
If the patient has significant difficulty instilling eye drops, a mechanical dosing aid may improve the likelihood of success. Adherence to the correct dosing schedule improves with the use of automated telecommunication-based reminders, smartphone and tablet based reminder apps.44,45 A combination of in-office education, goal setting, simplified drop regimen and technology should be embraced to improve adherence.46 Addressing known barriers to medication adherence is a necessary first step toward success.
Dropping the Pressure
IOP is the only known modifiable glaucomatous risk factor and lowering it has undeniably proven to reduce the risk of disease progression.47,48 Lowering IOP effectively thus becomes the goal when managing patients with glaucoma. After the disease is accurately classified, a therapeutic goal is typically set. Preferred practice patterns in the United States suggest the use of a target IOP range that the clinician believes will prospectively reduce the patients’ lifetime risk of blindness while concurrently minimizing treatment-related burden.49–52
Although multiple methodologies can help determine an initial target IOP (threshold IOP, calculated, one size fits all) the simplest and most evidence-based method is to reduce the IOP by a percentage from the baseline peak diurnal IOP at which damage to the optic nerve is occurring. IOP is dynamic and exhibits short-term and long-term fluctuations, which makes currently available tonometry not perfectly reproducible. Therefore, it is recommended that the clinician obtain multiple IOP readings at different times of day prior to initiating treatment to attempt establishing a diurnal peak IOP from which to base the target range.53-55 However, short of obtaining multiple 24-hour IOP curves, it is unlikely a true peak will ever be captured. The clinician will ultimately need to gauge successful treatment by reducing the rate of progression on structural and functional testing.48,56,57
Recommended target IOP percentage reductions range from 20% to 50% depending on the condition (i.e., glaucoma suspect, ocular hypertension, normal tension glaucoma, high tension glaucoma, angle closure glaucoma) baseline disease severity, and life expectancy.58-61
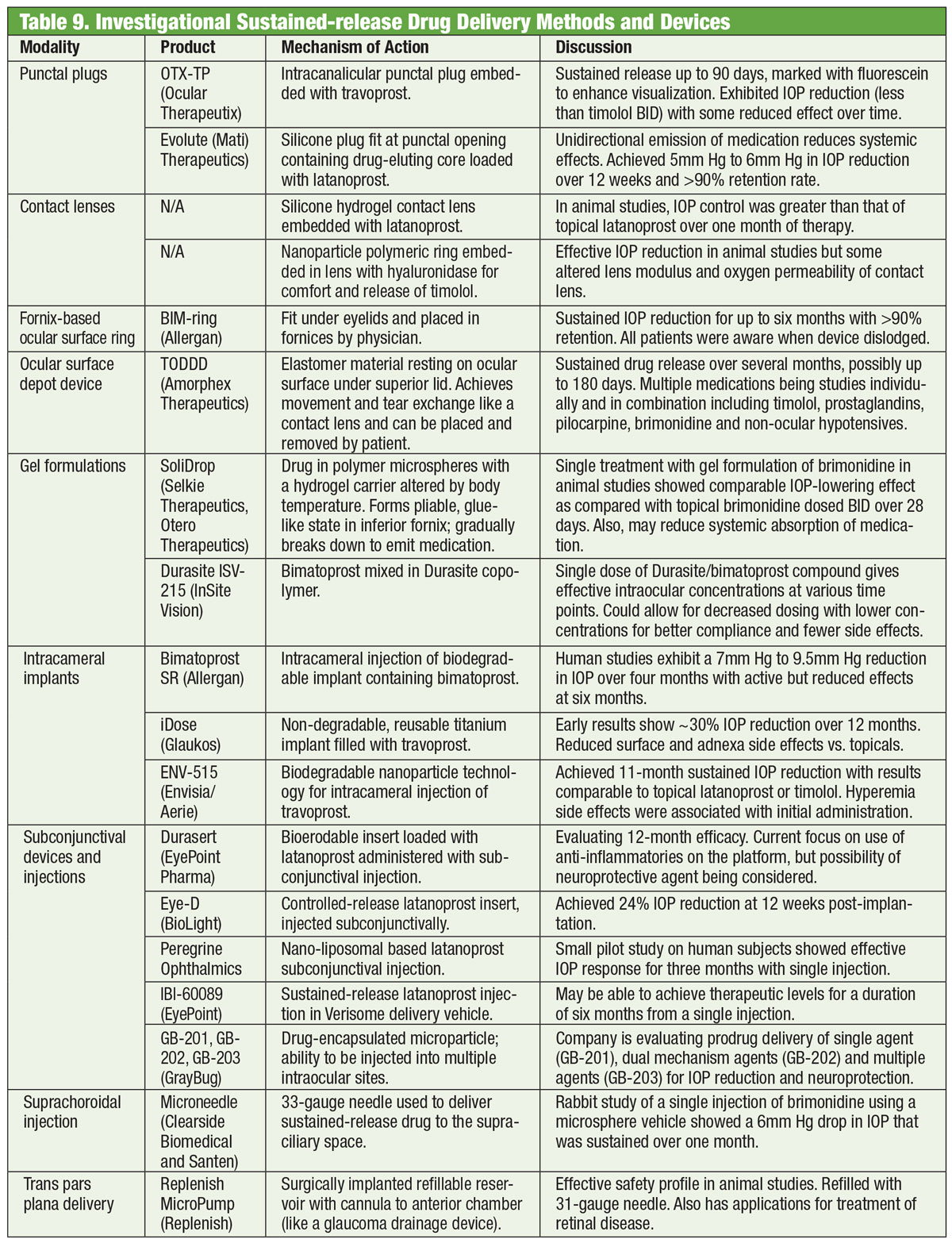 |
| Table: Bruce Onofrey, OD, RPh. Click image to enlarge. |
Getting Started
PGAs are approved for first-line treatment in the United States and are more effective in lowering IOP, have a relatively limited side effect profile and are dosed less frequently (once daily) than other classes of medication.62,63
Once a specific agent is selected, the patient should be seen again in no more than four weeks to gauge the effectiveness of the drop, to head off any adverse events that may arise, ensure patient adherence and reinforce the value of treatment.
Ultimately, proper education and vigilant follow-up with structural and functional testing will help minimize vision loss.
Dr. Dorkowski is the clinical coordinator for the Nursing Home/Assisted Living Program at SCO.
Dr. Williamson is the residency supervisor at the Memphis VA Medical Center.
Dr. Rixon is an attending at the Memphis VA and is a member of the Optometric Glaucoma Society.
Dr. Onofrey is an author of The Ocular Therapeutics Handbook.
|
1. Zimmerman TJ, William P. BogerIII. The beta-adrenergic blocking agents and the treatment of glaucoma. Surv Ophthalmol. 1979;23(6):347-62. 2. Realini T. A history of glaucoma pharmacology. Optom Vis Sci. 2011;88(1):36-8. 3. Erickson KA, Schroeder A. Direct effects of muscarinic agents on the outflow pathways in human eyes. Invest Ophthalmol Vis Sci. 2000;41(7):1743-8. 4. Drance S, Nash P. The dose response of human intraocular pressure to pilocarpine. Can J Ophthalmol. 1971;6(1):9-13. 5. Sears M. The mechanism of action of adrenergic drugs in glaucoma. Investig Opthalmology Vis Sci. 1966;5:115-9. 6. Mandell AI, Stentz F, Kitabchi AE. Dipivalyl epinephrine: a new pro-drug in the treatment of glaucoma. Ophthalmology. 1978;85(3):268-75. 7. Neufeld AH. Experimental studies on the mechanism of action of timolol. Surv Ophthalmol. 1979;23(6):363-70. 8. Coakes RL, Brubaker RF. The mechanism of timolol in lowering intraocular pressure: In the normal eye. Arch Ophthalmol. 1978;96(11):2045-8. 9. Trope GE, Clark B. Beta adrenergic receptors in pigmented ciliary processes. Br J Ophthalmol. 1982;66:788-92. 10. Wax MB, Molinoff PB. Distribution and properties of beta-adrenergic receptors in human iris-ciliary body. Invest Ophthalmol Vis Sci. 1987;28(3):420-30. 11. Allen RC, Hertzmark E, Walker AM, Epstein DL. A double-masked comparison of betaxolol vs timolol in the treatment of open-angle glaucoma. Am J Ophthalmol. 1986;101(5):535-41. 12. Novack GD. Ophthalmic beta-blockers since timolol. Surv Ophthalmol. 1987;31(5):307-27. 13. Soll DB. Evaluation of timolol in chronic open-angle glaucoma: Once a day vs twice a day. Arch Ophthalmol. 1980;98(12):2178-81. 14. Topper JE, Brubaker RF. Effects of timolol, epinephrine, and acetazolamide on aqueous flow during sleep. Invest Ophthalmol Vis Sci. 1985;26(10):1315-9. 15. Krag S, Andersen HB, Sorensen T. Circadian intraocular pressure variation with beta-blockers. Acta Ophthalmol Scand. 1999;77(5):500-3. 16. Schuman JS. Effects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Brimonidine Study Groups 1 and 2. Ophthalmology. 2000;107(6):1171-7. 17. Salim S, Shields MB. Glaucoma and systemic diseases. Surv Ophthalmol. 2010;55(1):64-77. 18. Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Retin Eye Res. 2000;19(1):87-112. 19. Loftsson T, Jansook P, Stefansson E. Topical drug delivery to the eye: dorzolamide. Acta Ophthalmol. 2012;90(7):603-8. 20. Lester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol. 2008;2(3):517-23. 21. Petounis A, Mylopoulos N, Kandarakis A, et al. Comparison of the additive intraocular pressure-lowering effect of latanoprost and dorzolamide when added to timolol in patients with open-angle glaucoma or ocular hypertension: a randomized, open-label, multicenter study in Greece. J Glaucoma. 2001;10(4):316-24. 22. Williams GC, Orengo-Nania S, Gross RL. Incidence of brimonidine allergy in patients previously allergic to apraclonidine. J Glaucoma. 2000;9(3):235-8. 23. Lee DA, Gornbein JA. Effectiveness and safety of brimonidine as adjunctive therapy for patients with elevated intraocular pressure in a large, open-label community trial. J Glaucoma. 2001;10(3):220-6. 24. Cantor LB, Safyan E, Liu C-C, Batoosingh AL. Brimonidine-purite 0.1% versus brimonidine-purite 0.15% twice daily in glaucoma or ocular hypertension: a 12-month randomized trial. Curr Med Res Opin. 2008;24(7):2035-43. 25. Beltz J, Zamir E. Brimonidine induced anterior uveitis. Ocul Immunol Inflamm. 2016;24(2):128-33. 26. Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53 Suppl1:S107-20. 27. Liu JHK, Slight JR, Vittitow JL, Scassellati Sforzolini B, Weinreb RN. Efficacy of latanoprostene bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am J Ophthalmol. 2016;169:249-57. 28. Kaufman PL. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin Pharmacother. 2017;18(4):433-44. 29. Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: The LUNAR study. Am J Ophthalmol. 2016;168:250-9. 30. Abu-Hassan DW, Acott TS, Kelley MJ. The trabecular meshwork: A basic review of form and function. J Ocul Biol. 2014. fulltextarticles.avensonline.org/JOCB-2334-2838-02-0017. Accessed June 29, 2018. 31. Sturdivant JM, Royalty SM, Lin C-W, et al. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorg Med Chem Lett. 2016;26(10):2475-80. 32. Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated iop treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-27. 33. Lin C-W, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1-2):40-51. 34. Shaw PX, Sang A, Wang Y, et al. Topical administration of a rock/net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp Eye Res. 2017;158:33-42. 35. Schmier JK, Hulme-Lowe CK, Covert DW. Adjunctive therapy patterns in glaucoma patients using prostaglandin analogs. Clin Ophthalmol. 2014;8:1097-104. 36. Fechtner BYRD, Khouri AS. Fixed combinations. Glaucoma Today. 2016;14(6)33-6. 37. Rossi G, Pasinetti G, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21(4):410-4. 38. Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Investig Ophthalmol Vis Sci. 2007;48(11):5052-7. 39. Jampel HD, Chon BH, Stamper R, et al. Effectiveness of intraocular pressure-lowering medication determined by washout. JAMA Ophthalmol. 2014;132(4):390-5. 40. Susanna R, De Moraes CG, Cioffi GA, Ritch R. Why do people (still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1. 41. Crabb DP. A view on glaucoma—Are we seeing it clearly? Eye. 2016;30(2):304-13. 42. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: A cross-sectional survey. Ophthalmology. 2015;122(7):1308-16. 43. Davis SA, Sleath B, Carpenter DM, Blalock SJ, Muir KW, Budenz DL. Drop instillation and glaucoma. Curr Opin Ophthalmol. 2018;29(2):171-7. 44. Boland M, Chang DS, Frazier T, et al. Automated telecommunication-based reminders and adherence with once-daily glaucoma medication dosing: The automated dosing reminder study. JAMA Ophthalmol. 2014;132(7):845-50. 45. Waisbourd M, Dhami H, Zhou C, et al. The Wills eye glaucoma app: Interest of patients and their caregivers in a smartphone-based and tablet-based glaucoma application. J Glaucoma. 2016;25(9):e787-e791. 46. Joseph A, Pasquale LR. Attributes associated with adherence to glaucoma medical therapy and its effects on glaucoma outcomes: An evidence-based review and potential strategies to improve adherence. Semin Ophthalmol. 2017;32(1):86-90. 47. Clement CI, Bhartiya S, Shaarawy T. New perspectives on target intraocular pressure. Surv Ophthalmol. 2014;59(6):615-26. 48. Sit AJ, Pruet CM. Personalizing intraocular pressure: Target intraocular pressure in the setting of 24-hour intraocular pressure monitoring. Asia-Pacific J Ophthalmol. 2016;5(1):17-22. 49. Jampel HD. Target pressure in glaucoma therapy. J Glaucoma. 1997;6(2):133-8. 50. Fingeret M. Care of the patient with open-angle glaucoma. Am Optom Assoc. 2011;1:1-161. 51. Singh K, Shrivastava A. Early aggressive intraocular pressure lowering, target intraocular pressure, and a novel concept for glaucoma care. Surv Ophthalmol. 2008;53(6 SUPPL.):33-8. 52. Prum Jr. BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma Preferred Practice Pattern guidelines. Ophthalmology. 2016;123(1):P41-P111. 53. Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 2010;117(9):1700-4. 54. Florent Aptel, MD, PhDemail, Antoine Lesoin, MSc, Christophe Chiquet, MD, PhD, Nishal Aryal-Charles, MSc, Christian Noel, MD, Jean-Paul Romanet M. Long-term reproducibility of diurnal intraocular pressure patterns in patients with glaucoma. Ophthalmology. 2014:1998-2003. 55. Rotchford AP, Uppal S, Lakshmanan A, King AJ. Day-to-day variability in intraocular pressure in glaucoma and ocular hypertension. Br J Ophthalmol. 2012;96(7):967-70. 56. Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124(6):793-7. 57. Konstas AGP, Quaranta L, Mikropoulos DG, et al. Peak intraocular pressure and glaucomatous progression in primary open-angle glaucoma. J Ocul Pharmacol Ther. 2012;28(1):26-32. 58. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701-30. 59. Heijl A, Cristina Leske M, Bengtsson B, et al. Reduction of Intraocular Pressure and Glaucoma Progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002 Oct;120(10):1268-79. 60. AGIS7. The advanced glaucoma intervention study (AGIS): The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2010;130:429-440. 61. Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the collaborative initial glaucoma treatment study. The impact of treatment and other baseline factors. Ophthalmology. 2009;116(2):200-207.e1. 62. Albert A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;(8):1967-85. 63. Stein JD, Shekhawat N, Talwar N, Balkrishnan R. Impact of the introduction of generic latanoprost on glaucoma medication adherence. Ophthalmology. 2015;122(4):738-47. 64. Weinreb RN, Ong T, Sforzolini BS, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: The VOYAGER study. Br J Ophthalmol. 2015;99(6):738-45. |

