 |
MIGS on the Move
A decade after their debut, these procedures now dominate the surgical sphere. Which options are most popular today, and where do ODs fit into the clinical picture?
By Jessica Steen, OD
Release Date: July 15, 2022
Expiration Date: July 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
Take the lead in the management of MIGS patients.
Recognize MIGS utility, device indications and efficacy.
Address and manage MIGS-related complications.
Comanage MIGS patients effectively.
Target Audience: This activity is intended for optometrists engaged in managing glaucoma patients with MIGS.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Jessica Steen, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #124283 and course ID 79159-GL. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Author: Dr. Steen has no relevant financial interests to disclose. Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
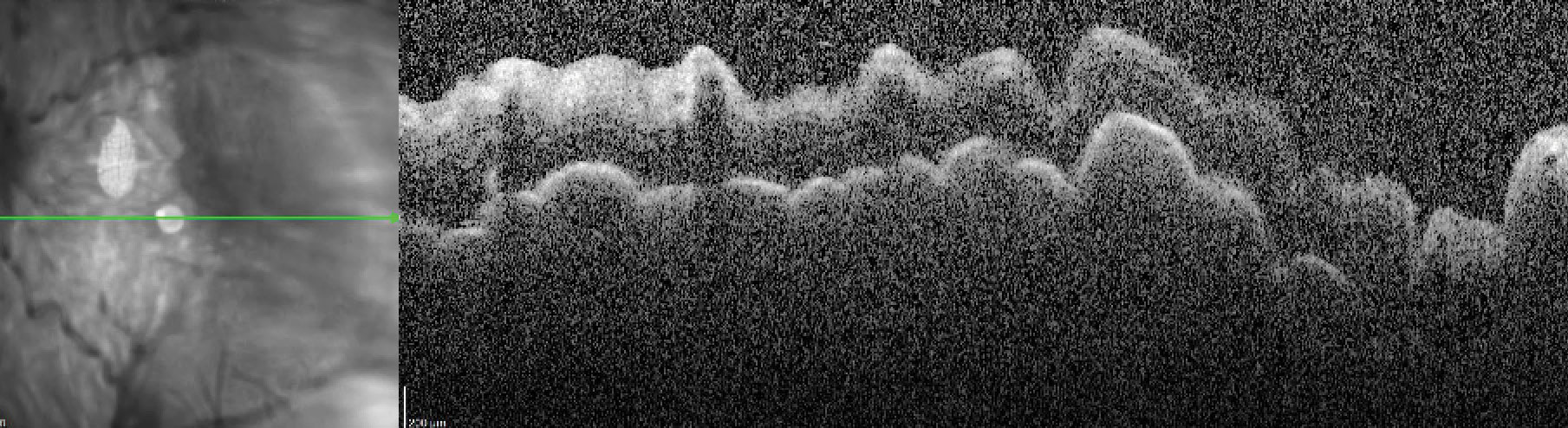 |
Hypotony maculopathy on spectral-domain OCT. Click image to enlarge. |
The traditional stepwise approach in the management of patients with open-angle glaucoma—moving from medication to laser-based procedures to surgical options, requiring failure at each step prior to moving to a successive level—has been disrupted through the development and improved understanding of efficacy and risk of available options.1-3 Glaucoma is the leading cause of global irreversible blindness, and despite continued research in disease pathogenesis, all available treatment options act to reduce intraocular pressure (IOP), the key treatable risk factor for disease development and progression.2-4
The number of glaucoma surgical procedures in the United States has rapidly grown over time, displaying a shift toward minimally invasive glaucoma surgery (MIGS) since regulatory approval of the first device in 2012 and a general reduction in traditional filtering procedures.3,4-7 While traditional incisional glaucoma procedures (i.e., trabeculectomy and aqueous shunt surgery), which create subconjunctival filtration blebs, can lower IOP substantially, they are subject to a significant recovery period and carry the risk of a wide variety of vision-threatening side effects.1-3
MIGS procedures act to bridge the gap between first-line options of medications and selective laser trabeculoplasty (SLT) and incisional or traditional glaucoma filtration procedures by lowering IOP while carrying a risk profile comparable to cataract surgery.1,2,8 While cataract surgery alone lowers IOP for a period of approximately two years, it is not recommended as a sole treatment for IOP reduction in individuals with primary open-angle glaucoma.1,8
In general, most MIGS devices and procedures are on-label for the treatment of mild to moderate open-angle glaucoma and ocular hypertension for patients undergoing cataract surgery.1,2 At this time, while not designed to be a replacement for the aggressive IOP reduction offered by traditional filtration procedures that may be required for advanced or refractory disease, MIGS can offer significant benefits in a widening spectrum of patients with open-angle glaucoma, including eliminating the need for IOP-lowering medication in patients with mild disease to reducing the number of medications required to delaying the need for incisional surgery in patients with more severe disease.1,2,6,9-11
MIGS devices and procedures vary in their location of impact and mechanism of IOP lowering but are generally characterized by these features: they are performed using an ab interno approach where no conjunctival incision is made, cause minimal trauma, are efficacious in lowering IOP with a high safety profile and are characterized by rapid recovery.9
Here, we discuss where MIGS stand today with a focus on understanding the physiological basis for MIGS utility, indications, efficacy and potential complications in order to optimize perioperative management.
 |
Two iStent inject devices. Image: Glaukos. Click image to enlarge. |
The MIGS Menu
These device options can be challenging to digest, but, in general, most surgeries act to reduce resistance to aqueous outflow through procedures which may or may not form a bleb.2,10,11 The surgical approach to bleb-forming MIGS may be ab interno or ab externo (outside of the eye); however, ab externo procedures do not fit the original criteria for MIGS and will not be included in this discussion.2,9-11
MIGS may enhance aqueous egress from the eye by increasing trabecular outflow through stripping, stenting or dilating tissue, enhancing uveoscleral outflow (via suprachoroidal pathways), creating a subconjunctival drainage pathway from the anterior chamber or reducing aqueous production.2,10,11 The latter (decreasing aqueous volume) can be accomplished through direct ablation of the ciliary process or application of energy to the ciliary body.2,10,11
• Among the many MIGS options, the first device to gain regulatory approval in the United States, the iStent trabecular microbypass stent, and its second-generation counterpart, the iStent inject, which has a modified design and consists of two stents, have accumulated the most diverse, long-term safety and efficacy data available. The iStent has been the most widely used device in the United States, accounting for 44% of glaucoma procedures in 2017.5,7
Recently released five-year data of the iStent inject shows sustained IOP-lowering in eyes as a standalone procedure and in eyes undergoing cataract surgery by an average of 42% and 39%, respectively, with 46% of patients medication-free and all eyes able to maintain or reduce their medication regimen over the study period, as well as an overall reduction in the need for IOP-lowering medication.12 Five eyes required additional surgical procedures for management of IOP.12
Postoperative findings of mild hyphema and mild corneal edema resolved within the first week.13 Two eyes of one standalone iStent inject patient developed cataract progression by postoperative month three, and an isolated eye from the standalone group developed anterior uveitis at month 24, which resolved with topical treatment.12 Over a five-year period, there were no stent-related complications, episodes of chronic inflammation, hypotony, endophthalmitis, Descemet’s membrane compromise, obstruction, myopic shift or choroidal detachment.12
• The Hydrus Microstent has a unique design that scaffolds Schlemm’s canal for approximately 90 degrees.14,15 Comparing the Hydrus Microstent implanted at the time of cataract surgery with cataract surgery alone, the Hydrus group had a higher proportion of eyes with IOP less than or equal to 18mm Hg without medication use, a lower average number of glaucoma medications, a greater percentage of eyes that were medication-free through five years and fewer eyes that required additional IOP-lowering procedures.15 There were no observations of late inflammation, corneal edema or need for device removal; however, focal peripheral anterior synechiae were identified near the trabecular entry point in 15% of the Hydrus Microstent group, which was not shown to have an impact on IOP during the study period.15
While no corneal edema or decompensation was identified through five years, reduction in corneal endothelial cell loss was identified in both the microstent plus cataract surgery group and the cataract surgery group alone at a similar rate with 21% of individuals exhibiting endothelial cell loss greater than or equal to 30% of baseline, a threshold considered as a significant change through five years with a stable rate of change noted over the study period.15 Endothelial cell loss following MIGS is of particular interest due to identified accelerated progressive endothelial cell loss previously noted following implantation of a supraciliary microstent, called CyPass, which resulted in voluntary market withdrawal.1,10,11
Aqueous Humor Dynamics for the ClinicianMIGS procedures take a more physiological approach to IOP lowering in comparison to incisional procedures or to aqueous suppressants.2,9 To best understand where and how specific MIGS options act to lower IOP and why post-op complications may arise, let’s briefly revisit aqueous humor dynamics with a specific focus on glaucoma pathology. Once aqueous humor is produced by the epithelia at the tips of the ciliary process and flows into the anterior chamber, it leaves the eye through the trabecular (conventional) and uveoscleral (alternate) pathways.13 In the conventional pathway, which accounts for as much as 90% of outflow, aqueous humor crosses the superficial portions of the trabecular meshwork, moves through the corneoscleral meshwork into the juxtacanalicular tissue and passes through the inner wall of Schlemm’s canal, where it encounters the greatest resistance.13 Once aqueous crosses into Schlemm’s canal, it enters one of approximately 30 external collector channels, which are distributed unevenly—with the greatest number of collector channels found in the inferior nasal quadrant of the eye—and flows into the deep scleral plexus and the episcleral venous system.13 When IOP increases, such as in the majority of eyes with open-angle glaucoma, the juxtacanalicular tissue and inner wall of Schlemm’s canal herniate into the ostia of collector channels, reducing their outflow capacity and further driving outflow resistance and elevating IOP.13 These occluded ostia seem to be of specific relevance to glaucoma treatment as glaucomatous eyes have a higher number of occluded collector channel ostia in comparison to nonglaucomatous eyes.13 While currently available MIGS procedures use a number of strategies for lowering IOP, including increasing trabecular and uveoscleral outflow and reducing aqueous production, the most commonly encountered procedures are aimed at the trabecular meshwork and Schlemm’s canal to allow the surgeon to directly treat the areas of the eye where outflow resistance is greatest in order to restore the conventional pathway’s outflow capacity.2,3,7,10,11 |
• No-device trabecular bypass, or excimer laser trabeculostomy, uses a 308nm xenon chloride excimer laser to create 10µm to 200µm trabeculostomy openings across 90° of the angle through the trabecular meshwork and inner wall of Schlemm’s canal.16 Properties of the excimer laser that make it attractive for intraocular use include the photoablative effect with minimal thermal damage and tissue penetration.16 While approved in the European Union, a clinical trial in the United States is currently recruiting patients to evaluate safety and efficacy of the procedure.17
• Dilation of Schlemm’s canal, or ab interno canaloplasty, resulting in breaking herniations in distal channels may be performed by flushing the canal with viscoelastic, threading a microcatheter or suture through the canal or a combination of the two procedures depending on the proprietary device used.18,19 Following threading of the microcatheter or suture through the canal, it may be pulled toward the center of the pupil to cleave the trabecular meshwork resulting in trabeculotomy.20
While published data exists to support IOP lowering, safety and mechanism of action based on physiological understanding of outflow pathways, long-term data and randomized controlled clinical trials are lacking.11 Mild postoperative hyphema is the most common complication encountered due to the necessary goniotomy needed to enter the canal, and risk of hyphema increases when trabeculotomy is performed.10,11,21
• Stripping procedures using devices such as the Trabectome and the Kahook Dual Blade remove a portion of the trabecular meshwork and inner wall of Schlemm’s canal to facilitate increased trabecular outflow.10,11,21,22 In a meta-analysis of 5,091 individuals who underwent Trabectome as a standalone procedure or combined phaco-Trabectome, the average IOP reduction was approximately 31% with final IOP near 15mm Hg and a reduction of IOP-lowering medication by less than one drop on average.21
The overall vision-threatening complication rate was less than 1%, with the most common complication identified as hyphema, which occurred up to 31 months following the procedure and was associated with transient IOP elevation.21 Peripheral anterior synechiae was reported in as many as 14% of cases.21 Less common reported complications included cyclodialysis cleft, which was identified in six instances, with one requiring surgical closure, transient hypotony (no cases persisted beyond three months) and aqueous misdirection syndrome.21 In patients undergoing Kahook Dual Blade, which may be performed as a standalone procedure or in combination with cataract surgery, reduction in IOP and postoperative medications is expected with a similar risk profile and complications in comparison to Trabectome.11,22
• In the case of refractory open-angle glaucoma, where previously surgical treatment has failed or where further IOP lowering is required beyond maximally-tolerated medical therapy, the Xen gel stent implant may be considered.1,11,23 Xen is sometimes compared with trabeculectomy as both are filtration bleb-dependent procedures, use mitomycin C intraoperatively, have similar post-procedure follow-up care and treatment and carry a similar IOP-lowering effect, albeit Xen carries an improved safety profile.24,25
Conversely to trabeculectomy, the Xen gel stent does not involve incision of the sclera and conjunctiva with on-label ab interno placement and does not require iridectomy or sutures.25 Xen differs from the majority of MIGS procedures in its indication due to its mechanism of IOP lowering by passing the physiological outflow pathways, potential for IOP lowering by an increased risk of complication and need for close postoperative observation by the operating surgeon due to the likelihood of post-procedure intervention.23-25
Comparison of success rates between MIGS presents challenges due to differences in patient characteristics, glaucoma subtype, follow-up duration, medication use, previous failed surgeries and definition of “success.” However, following Xen implantation, up to 89% of eyes had IOP less than or equal to 21mm Hg or 20% lower than baseline without requiring additional medical therapy at one year and 71% at two years, with a significant reduction in the number of post-procedure IOP-lowering medications.24 Overall, IOP was reduced by 35% to a final average near 15mm Hg with a greater IOP-lowering effect identified in eyes with higher baseline IOP in the included studies which ranged in duration from 12 to 36 months.24
Postoperative management of patients undergoing Xen implantation is more complex than other MIGS procedures due to the risk of complications and need for additional intervention. To limit the risk of subconjunctival fibrosis, bleb needling is considered to be a routine part of postoperative care, with most eyes undergoing needling within one month following surgery and some requiring subconjunctival 5-fluorouracil treatment.24,25
Hypotony, or IOP less than 6mm Hg, may occur in up to 10% of eyes and is typically described to be transient with improvement within one month.24 Hypotony-related complications include maculopathy and choroidal effusion.23-25 Hyphema was generally mild (less than one-third of the anterior chamber) and is reported to occur in 6% of eyes which may result in transient IOP spikes.24,25 Other complications following Xen implantation related to the device include occlusion, exposure, migration and bleb-related complications such as bleb leakage, blebitis and endophthalmitis.23-25
• Reduction in aqueous humor production using micropulse transscleral laser therapy carries a lesser risk of hypotony, pupillary mydriasis, chronic inflammation and vision loss in comparison to standard cyclodestructive thermal procedures such as endocyclophotocoagulation and transscleral cyclophotocoagulation.26
Micropulse transscleral laser therapy delivers energy to the pars plana (rather than to the pars plicata) in a series of repetitive, short pulses with incorporated rest periods to prevent reaching a cyclodestructive threshold.26 It is presumed to reduce IOP through biological stimulation resulting in increased trabecular and uveoscleral outflow in addition to aqueous suppression.26 Micropulse transscleral laser therapy reduces IOP gradually, with maximum IOP lowering of 31% achieved at 12 months.26
 |
Hydrus microstent placed within Schlemm’s canal. Photo: Justin Schweitzer, OD. Click image to enlarge. |
Collaborative Pre-, Post-op Care
Based on the variety of procedures available and an understanding of the indications, general success rate and potential complications of MIGS, effective communication with the consultative surgeon is central to maximizing outcomes.
At the time of referral, providing the surgeon with the patient’s glaucoma and ocular history—including glaucoma diagnosis (which requires careful gonioscopic evaluation), disease severity, treatment history, the general roadmap of IOP (peak, untreated pressure if available and target pressure)—and patient goals will help the surgeon in determining the optimal procedure.
If cataract is present, evaluation to determine the presence of ocular findings that may increase the complexity of surgery (e.g., peripheral anterior synechiae, conjunctival scarring, zonular instability) is necessary.2 While all procedures carry risks, MIGS vary in their potential IOP-lowering effect, safety profile and indications, all of which are carefully considered as part of the complex decision-making process in determining a procedure of choice.2,27,28
When asked which IOP-lowering procedure American Glaucoma Society members would choose for themselves as the patient—following need for further IOP lowering after SLT and maximizing all commercially available topical glaucoma medications—the most frequent responses, in order, were ab interno trabeculotomy, Xen gel stent and iStent inject, followed by trabeculectomy.29 With traditional trabeculectomy being preferred by participants older than 61, in the youngest cohort of participants (between 30 and 40 years of age), the top four ranked procedures were all forms of MIGS, with trabeculectomy being ranked lower.29
The procedures that surgeons may choose as a primary procedure for themselves and those they are most likely to offer a hypothetical patient in a similar setting differed.3,29 When analyzing surgical practice preference, 59% of individuals would offer a hypothetical patient traditional trabeculectomy with mitomycin C in 2016, where only 18% would prefer to have the same procedure performed on themselves in 2018.3,29 The short time period between the two studies and varying results further adds support to reflect the rapid paradigm shift in consideration of MIGS that has occurred in the United States.
Due to the relatively recent development and use of MIGS, the long-term outcomes, generally beyond five years, are not known.1,2 Successful postoperative care requires a collaborative relationship with direct communication between the comanaging optometrist and surgeon. Postoperatively, patients can be expected to use the typical regimen of topical ocular medications, including prophylactic antibiotic, nonsteroidal anti-inflammatory and steroid taper, over a four-week period.27,28
For individuals with mild to moderate disease undergoing MIGS strategies that target Schlemm’s canal and the trabecular meshwork, IOP-lowering medications may not be stopped prior to surgery. A reduction in IOP-lowering therapy can be considered as early as one week post-op if target pressure is achieved.
Due to the risk of hypotony in the immediate postoperative period, patients undergoing bleb-forming MIGS should have IOP-lowering medications discontinued on the day of the procedure.24,25 The most common expected postoperative course includes mild inflammation and hyphema appearing as an anterior chamber reaction resulting in blurred vision, which is typically mild and self-limiting within the first postoperative week.28
Fluctuations in IOP postoperatively can be a sign of increased inflammation, hyphema, device malposition or obstruction, wound leak and cyclodialysis cleft.27,28 Careful clinical examination to determine the cause followed by conservative treatment with topical steroids, cycloplegics or IOP-lowering medications may be needed. In the case of device malposition, obstruction, significant or recurrent hyphema or sustained IOP elevation, the patient should be promptly evaluated by the surgeon.30
Hypotony following a canal-based procedure in the absence of wound leakage should be suspicious for cyclodialysis cleft, as these procedures do not bypass the conventional pathway. Therefore, aqueous must still overcome episcleral venous pressure to leave the eye, preventing IOP from dropping below episcleral venous pressure. Iatrogenic cyclodialysis cleft, or separation of the longitudinal fibers from the scleral spur during surgery, results in an unintentionally produced drainage pathway of aqueous from the anterior chamber directly into the suprachoroidal space.31
The mainstay of medical treatment for hypotony due to cyclodialysis cleft is cycloplegia to allow the ciliary muscle to relax and detached fibers to reattach to the scleral spur with consideration of increase in topical ocular steroid dosage.31 If conservative treatment does not close the cleft, surgical or laser therapy may be employed.31
While MIGS are characterized by their rapid recovery and high safety profile, patients do experience postoperative complications. While generally mild, transient and self-limiting, depending on the procedure, they may occur at a similar or higher rate than cataract surgery alone.28 If a complication is identified during the post-op course, the comanaging physician should have a low threshold for intervention, and when resolution does not occur with conservative treatment, they should have the patient return to the surgeon.
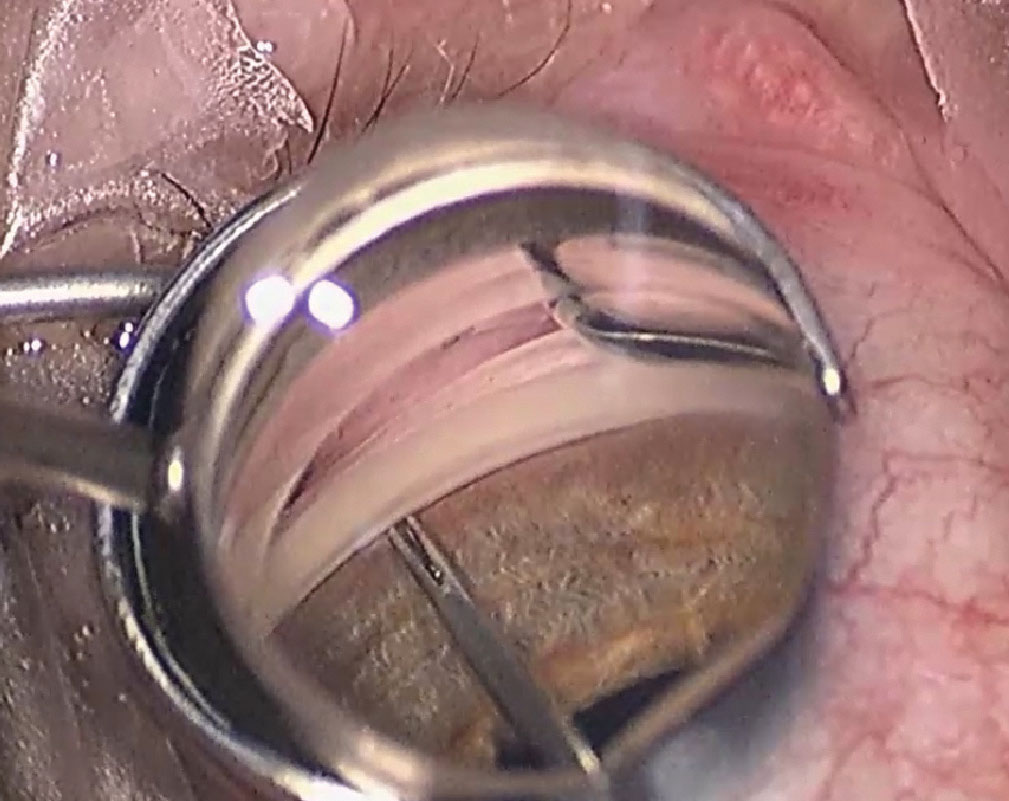 |
Placement of iStent inject perpendicular to the trabecular meshwork. Photo: Justin Schweitzer, OD. Click image to enlarge. |
The Bottom Line
Developments in the MIGS space focus primarily on enhancement of natural physiology to reduce IOP. While not a replacement for incisional glaucoma surgeries, such as trabeculectomy or aqueous shunts, a variety of MIGS have established their safety and efficacy as a bridge between first-line therapies (i.e., medications and SLT) and traditional filtering procedures. MIGS continue to expand their reach with available and developing devices, challenging the IOP-lowering potential of filtration procedures with the goal of an improved safety profile.
Comanaging providers should be familiar with available procedures and devices as well as expected results, including complications, and take a collaborative approach to pre- and post-op care. Randomized controlled clinical trials evaluating MIGS in a standardized way and evaluation of long-term durability are ongoing as the MIGS experience continues to develop.
Dr. Steen is an attending optometrist and assistant professor at Nova Southeastern University College of Optometry. She has no financial interests to disclose.
1. Weinreb RN, Garway-Heath DF, Leung C, et al. Glaucoma Surgery: WGA consensus series – 11. 2019. Amsterdam, The Netherlands: Kugler Publications. 2. Fellman RL, Mattox C, Singh K, et al. American Glaucoma Society Position Paper: Microinvasive Glaucoma Surgery. Ophthalmol Glaucoma. 2020;3:1-6. 3. Vinod K, Gedde SJ, Feuer WJ, et al. Practice Preferences for Glaucoma Surgery: A Survey of the American Glaucoma Society. J Glaucoma. 2017;26:687-93. 4. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and metaanalysis. Ophthalmology. 2014;121:2081e90. 5. Lee JH, Ma AK, Warren JL, et al. Impact of iStent Micro-Bypass Shunt on Medicare Part B Glaucoma Surgical Expenditure. Ophthalmol Glaucoma. 2021;4:131-8. 6. Boland MV, Corcoran KJ, Lee AY. Changes in Performance of Glaucoma Surgeries 1994 through 2017 Based on Claims and Payment Data for United States Medicare Beneficiaries. Ophthalmol Glaucoma. 2021;S2589–4196:00032–6. 7. Yang SA, Mitchell W, Hall N, et al; IRIS® Registry Data Analytics Consortium. Trends and Usage Patterns of Minimally Invasive Glaucoma Surgery in the United States: IRIS® Registry Analysis 2013-2018. Ophthalmol Glaucoma. 2021;4:558-68. 8. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119:1826-31. 9. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96-104. 10. Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma serieies (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142. 11. Nichani P, Popovic MM, Schlenker MB, et al. Microinvasive glaucoma surgery: A review of 3476 eyes. Surv Ophthalmol. 2021;66:714-42. 12. Hengerer FH, Auffarth GU, Conrad-Hengerer I. iStent inject Trabecular Micro-Bypass with or Without Cataract Surgery Yields Sustained 5-Year Glaucoma Control. Adv Ther. 2022;39(3):1417-31. 13. Freddo T, Civan M, Gong H, et al. Aqueous humor and the dynamics of its flow: mechanisms and routes of aqueous humor drainage. Albert and Jakobiec’s Principles and Practices of Opthalmology. 4th edition. Springer. 14. Samet S, Ong JA, Ahmed II. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis (Lond) 2019; 6:32. 15. Ahmed IIK, De Francesco T, Rhee D, et al. HORIZON Investigators. Long term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022:S0161-6420(22)00160-9. 16. Durr GM, Töteberg-Harms M, Lewis R, et al. Current review of Excimer laser Trabeculostomy. Eye Vis (Lond). 2020;7:24. 17. Elios Vision, Inc. Excimer laser trabeculostomy glaucoma treatment study (ELTGTS). https://clinicaltrials.gov/ct2/show/NCT04899063. Retrieved April 2, 2022. 18. New World Medical announces launch details for the STREAMLINE Surgical System. 2022. https://www.newworldmedical.com/new-world-medical-announces-launch-details-for-the-streamline-surgical-system/. 19. Gallardo MJ. 36-Month Effectiveness of Ab-Interno Canaloplasty Standalone versus Combined with Cataract Surgery for the Treatment of Open-Angle Glaucoma. Ophthalmol Glaucoma. 2022:S2589-4196(22)00025-4. 20. Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma 2018;27:393-401. 21. Kaplowitz K, Bussel II, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100(5):594-600. 22. Iwasaki K, Kakimoto H, Orii Y, et al. Long-Term Outcomes of a Kahook Dual Blade Procedure Combined with Phacoemulsification in Japanese Patients with Open-Angle Glaucoma. J Clin Med. 2022;11(5):1354. 23. Allergan, Inc. XEN Gel Stent (Instructions for Use), Irvine, CA: Allergan, Inc.; 2017. https://allergan-web-cdn-prod.azureedge.net/actavis/actavis/media/allergan-pdf-documents/labeling/xen/dfu_xen_glaucoma_treatment_system_us_feb2017.pdf. 24. Chen XZ, Liang ZQ, Yang KY, et al. The Outcomes of XEN Gel Stent Implantation: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2022;9:804847. 25. Lewczuk K, Konopińska J, Jabłońska J, et al. XEN Glaucoma Implant for the Management of Operated Uncontrolled Glaucoma: Results and Complications during a Long-Term Follow-Up. J Ophthalmol. 2021;2021:2321922. 26. Bernardi E, Töteberg-Harms M. MicroPulse Transscleral Laser Therapy Demonstrates Similar Efficacy with a Superior and More Favorable Safety Profile Compared to Continuous-Wave Transscleral Cyclophotocoagulation. J Ophthalmol. 2022;2022:8566044. 27. Aptel F, Colin C, Kaderli S, et al. Management of postoperative inflammation after cataract and complex ocular surgeries: a systematic review and Delphi survey. Br J Ophthalmol. 2017;101:1-10. 28. Yook E, Vinod K, Panarelli JF. Complications of microinvasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29:147-54. 29. Chang TC, Vanner EA, Parrish RK 2nd. Glaucoma surgery preferences when the surgeon adopts the role of the patient. Eye (Lond). 2019 Oct;33:1577-83. 30. Siedlecki A, Kinariwala B, Sieminski S. Uveitis-Glaucoma-Hyphema Syndrome Following iStent Implantation. Case Rep Ophthalmol. 2022;13(1):82-8. 31. Ioannidis AS, Barton K. Cyclodialysis cleft: causes and racepair. Curr Opin Ophthalmol. 2010;21(2):150-4. |
