 |
A New Consensus on Keratoconus
Collagen crosslinking has prompted a more interventional approach to this condition.
By Mitch Ibach, OD
Release Date: April 15, 2021
Expiration Date: April 15, 2024
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group
Educational Objectives: After completing this activity, the participant should be better able to:
- Review the impact of advancing diagnostics.
- Discuss the current standard of care for keratoconus.
- Recognize the role of corneal crosslinking.
- Determine when CXL is an appropriate treatment option.
Target Audience: This activity is intended for optometrists engaged in routine eye care and ocular surface disease management.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Jennifer Gould, OD, SUNY College of Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 71975-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Author: Dr. Ibach receives consulting fees, fees for non-CME/CE services and conducts research with Glaukos.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
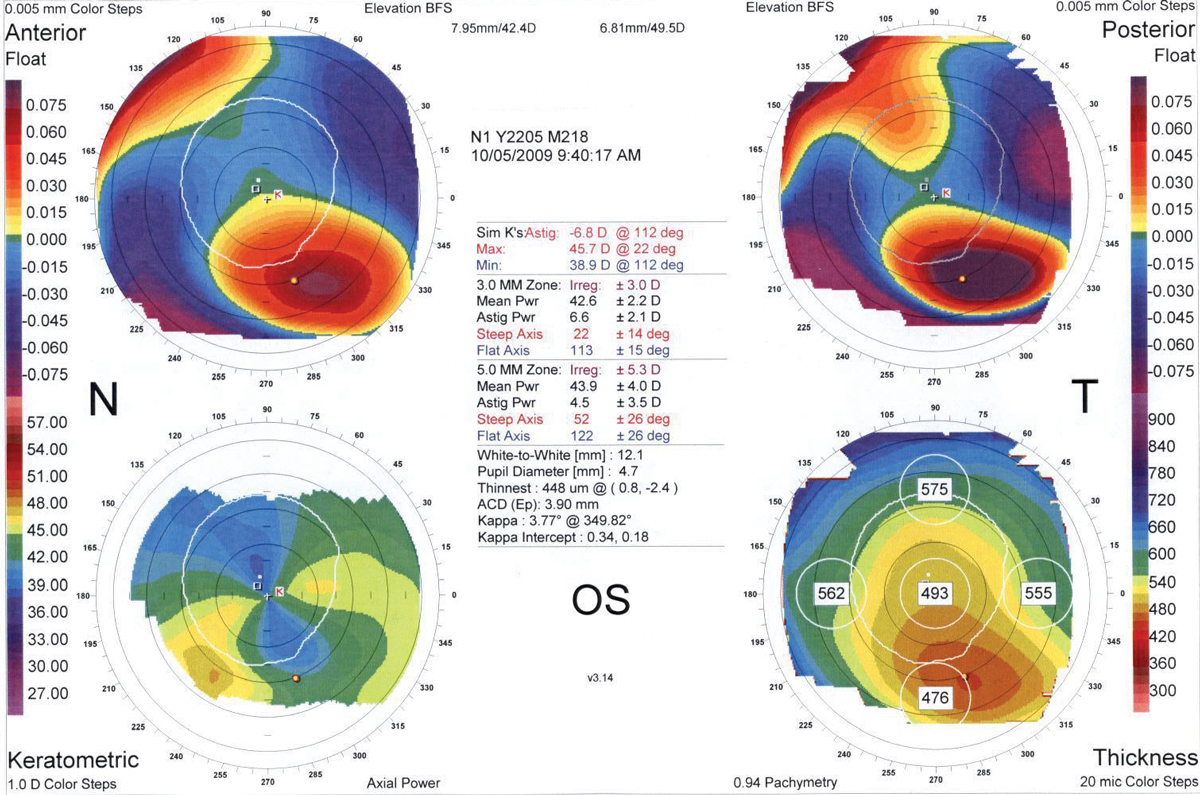 |
| Fig. 1. Corneal topography showing a classic inferior steepening to the anterior float. Note the thinned corneal pachymetry in the bottom right. Click image to enlarge. |
Imagine encountering a progressive, vision-threatening ocular disease like glaucoma and recommending a treatment plan focused on monitoring, while temporarily improving acuity with glasses and contact lenses, and reserving treatment for only the most end-stage cases. Up until five years ago, this was the standard management approach for patients with keratoconus.
As eye care providers, how do we stop keratoconus, a disease that causes irreversible vision loss, before it gets to the final surgical option? This article presents a new paradigm in keratoconus management that focuses on early diagnosis and earlier intervention, to first catch and then halt this ocular disease.
The standard of care for keratoconus management should shift away from a “monitoring” approach and instead embrace an “interventional” one. The new mantra should prioritize new technology to facilitate earlier diagnosis united with early treatment to stop disease progression. The current staple in halting disease progression by strengthening the corneal biomechanics is corneal collagen crosslinking (CXL).
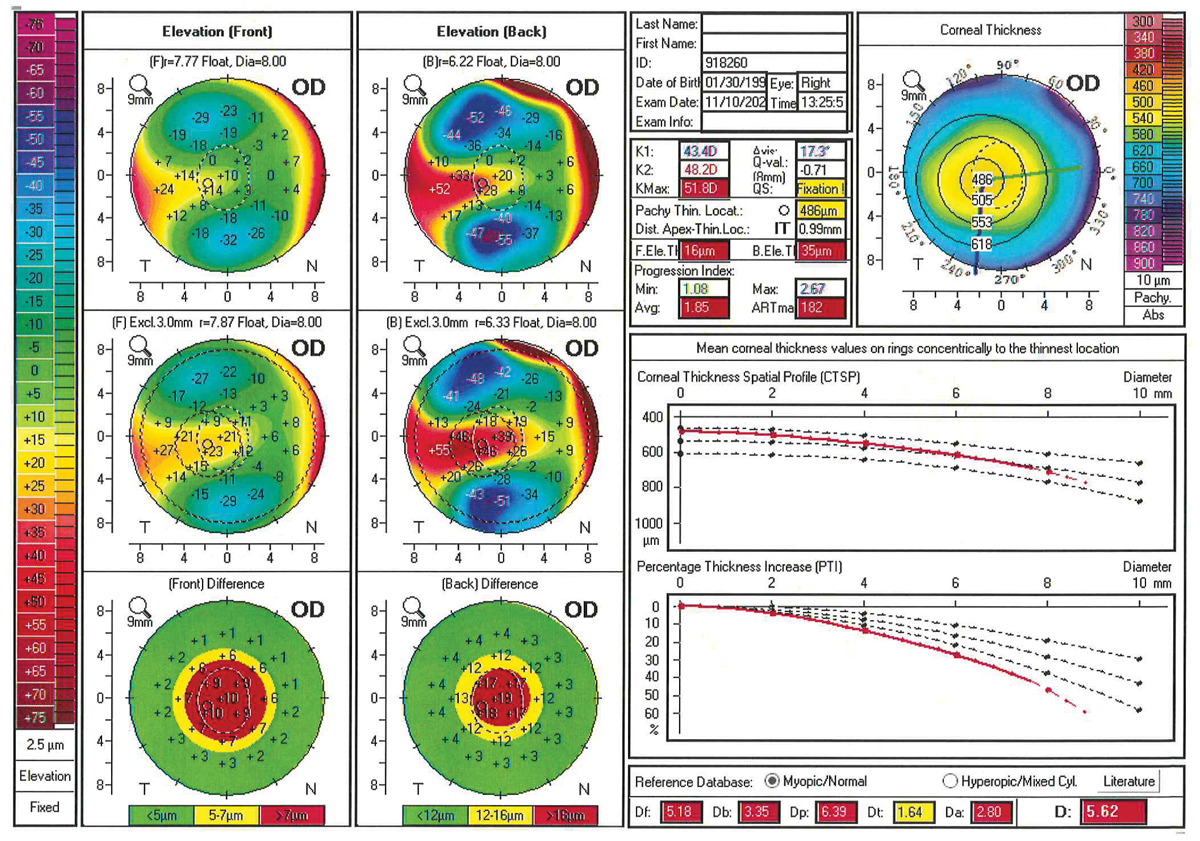 |
Fig. 2. Pentacam Belin-Ambrosio Enhanced Ectasia Display showing early keratoconus. Note how the corneal percentage thickness increase (lower right graph) is outside normal limits. Also, the color-coded “front” and “back” differences, comparing this patient to a best-fit sphere, are a cause for concern. Click image to enlarge. |
Once the cornea is stabilized, this opens the practitioner’s refractive toolbox of glasses, contact lenses, intrastromal corneal ring segments (ICRS) and topography-guided photorefractive keratectomy. Optometrists play a key role in all three phases of the keratoconus patient life cycle, starting with diagnosis, because many keratoconus patients initially present to optometric practices. After stabilizing treatment (second phase), optometrists are the key providers for optical rehabilitation, the final phase.
Prompt Diagnosis
Advancements in diagnostic technologies have simplified diagnosis for practitioners, especially in the early or pre-clinical keratoconus patient. Even if your practice doesn’t have these new tools, you can still be a master diagnostician. For example, honing in on a “scissoring” reflex with retinoscopy can approach tomography in sensitivity and specificity for diagnosis.1 In keratoconus patients, the retinoscope reflex bending or bowing is a sign of corneal irregularity. Critically analyzing refractive increases in myopic spherical equivalent and or cylinder increases can raise red flags for early ectasia. An adolescent patient who has yearly continued increases in myopic spherical equivalent greater than 1.00D deserves further testing.
What is Keratoconus? The condition is a non-inflammatory, bilateral, often asymmetric corneal degeneration that leads to corneal thinning and steepening (ectasia). In the early stages, patients will lose uncorrected visual acuity, followed by increases in myopic spherical equivalent and regular astigmatism, and finally lose best-corrected visual acuity (BCVA) due to irregular corneal astigmatism. The prevalence of keratoconus is debatable and inarguably increasing in published literature and among several demographic groups. A now outdated but heavily referenced study for US rates of keratoconus revealed that about one in 1,835 people were affected by the disease.25 More recent global estimates of keratoconus reported a worldwide prevalence of about one in 750.26 Most recently, the Raine Study looked at a cohort of 1,259 patients from Western Australia and found that the prevalence of keratoconus to be 1.2%, or one out of every 84 people.27 This rise in reported prevalence isn’t because keratoconus has suddenly become transmissible or contagious, but rather is likely due to improved diagnostic technologies and increased patient awareness driven by the availability of new treatment options. A keratoconus patient’s risk profile can vary based on family history and environmental factors. Overall, the disease shows weak genetic linkages in both autosomal dominant and autosomal recessive genes.5,28,29 First, systemic diseases showing increased risk of keratoconus include Down syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta and mitral valve prolapse among others to a lesser extent.29,30 Studies have shown that patients with Down syndrome have anatomically thinner and steeper corneas, an increased likelihood of aggressive eye rubbing and a keratoconus prevalence at least 10 times higher than non-Down syndrome patients.30 Mechanical eye-rubbing is a modifiable risk factor for the development and progression of keratoconus.29,30 Ocular atopies (including allergic/vernal conjunctivitis and atopic dermatitis), floppy eyelid syndrome, dry eye disease, blepharitis, digital eyestrain and poorly fitting contact lenses can all lead to the stimulus to rub. Treating these comorbid ocular conditions is an important adjunctive approach for keratoconus. The painless nature of keratoconus, combined with the relative ease of maintaining adequate visual acuity with stronger optical devices, may cause delays in the detection of ectasia. |
Diagnostic technology can be subdivided into tools for definitive diagnosis where clinical keratoconus is present and diagnostic tools for pre-clinical disease. Assessing corneal curvature with topography and tomography is a mainstay in keratoconus management. A non-symmetric elevation pattern or bowtie, most commonly displaying inferior steepening on topography, is pathognomonic for corneal ectasia (Figure 1). Corneal topography is an analysis of the anterior corneal curvature which reports two simulated keratometry values (steep and flat K), corneal astigmatism and the corneal shape/symmetry pattern. Topographers most commonly apply placido disc imaging techniques, but scanning slit technique (Orbscan, Bausch + Lomb) does allow posterior corneal measurements.
Advanced technology is giving practitioners more sensitive data that can allow for earlier interventions. While these new tools are valuable additions to the optometrist’s arsenal, clinicians can still diagnose keratoconus even if they do not have access to these technologies. Corneal tomographers employ Scheimpflug imaging which uses a rotating camera to analyze slit beams at different angles.2 This technique is best suited for a non-planar surface like the cornea.
Corneal tomography summarizes anterior corneal shape, posterior corneal shape and corneal pachymetry mapping, and can enroll advanced analyses specifically designed for corneal ectasias like the Belin-Ambrosio Enhanced Ectasia Display (Figure 2). Tomography has become the diagnostic gold standard for management of the irregular cornea in today’s research and clinical practice.3 Anterior segment optical coherence tomography (AS-OCT) is another tool that fits more likely as an adjunctive diagnostic for definitive keratoconus diagnosis. AS-OCT provides a high-definition cross-section of the corneal shape.
In the pursuit of earlier diagnosis, evolving tools for pre-topographic detection of keratoconus are gaining traction. Epithelial mapping uses specialized OCT, which is able to measure and map the most anterior corneal layer. Specifically comparing keratoconic eyes vs. normal, a keratoconus patient’s exhibit thinner central epithelium, thinner minimum epithelial thickness, thinner inferior temporal epithelium and a greater difference in superior to inferior epithelial mapping.4 Summarizing these findings, the epithelium drifts toward thinning directly over the area of posterior corneal bulging, which corresponds to the apex of the cone, and can precede topographic findings.4
Although familial history shows a scattered genetic pattern, patients with a positive keratoconus familial history are at a higher risk of developing the disease.5 Having a first-degree relative with keratoconus is an established risk factor for the disease.6 Thus, genetic testing is a blossoming method for determining a patient’s risk. AvaGen (Avellino Labs) is an in-office cheek swab that examines 75 genes with over 1,000 variants to quantify a patient’s relative risk for keratoconus.7 In genetically high-risk patients, this data may spur practitioners and patients to tighten their follow-up schedule, seek genetic counseling or consider interventional treatment at an earlier stage.
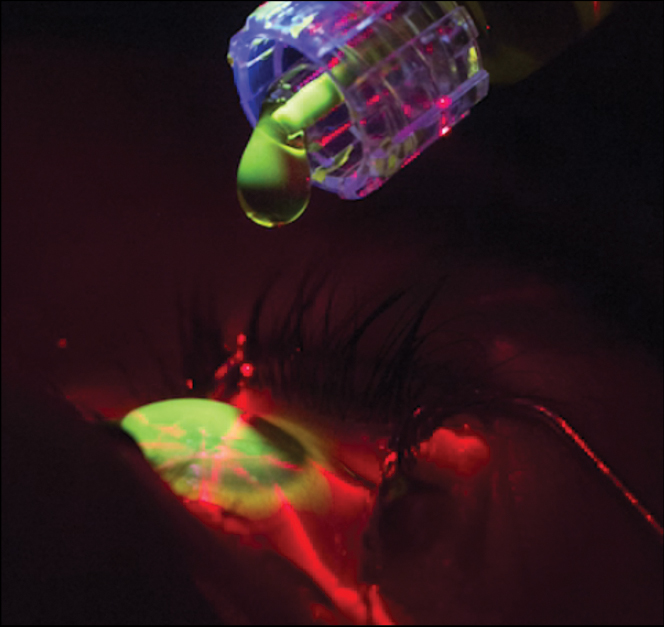 |
|
Fig. 3. Procedural view during CXL. Click image to enlarge. |
Early Intervention
After diagnosis, the next step in keratoconus management is to prevent further progression. Currently, the only treatment aimed at halting corneal ectasia progression with long-term efficacy is CXL. Optometrists play a key role in pre-op education and referral. They are also instrumental in collaborative care including vision rehabilitation with optical devices.
A timely referral for intervention is critical because vision loss due to corneal warpage is often irreversible. In many cases as an early keratoconus patient progresses, changing the prescription in glasses or contact lenses can improve acuity but mask worsening disease. Since patients aren’t born with the disease, all keratoconus patients had progression at one point, but younger patients and patients who are aggressive eye rubbers have been shown to have a greater risk of keratometry steepening/worsening.8 The right patient for CXL is any patient showing progression to this ectatic disease who can safely receive treatment.
Concerning the question, “Which comes first: crosslinking or specialty contact lenses?” I prefer to crosslink these corneas first. After the cornea has been stabilized, specialty contact lenses should provide years of improved visual acuity. However, if a patient has reduced visual function at work or is unable to drive due to vision loss, a specialty contact lens may bridge the gap and greatly improve their quality of life. It’s imperative to remember despite improved visual acuity, these patients still have a biomechanically unstable cornea.
 |
Fig. 4. Example of progression parameters that can be helpful for insurance reimbursement. Click image to enlarge. |
The main contraindication to CXL is pregnancy. This patient subset was not studied in the FDA clinical trials.9 Relative contraindications include individuals under the age of 14 or older than 65 years.9 More commonly patients under age 14, but in both groups, CXL has been safely performed and can be of tremendous benefit. Patients with active infectious keratitis should be avoided as this is currently off-label, but studies suggest potential benefit and are ongoing.
Epithelium-off CXL was approved by the FDA in 2016 for the treatment of progressive keratoconus and post-refractive surgery ectasia. CXL is a medical procedure that combines riboflavin (vitamin B2) photosensitizer with ultraviolet light (365nm to 370nm) to stiffen the cornea. The coupling of riboflavin and UVA light forms free radicals and singlet oxygen molecules creating covalent bonds or “crosslinks” in the corneal lamellae.10,11 This chemical reaction leads to a shortening, thickening and stiffening of the corneal tissue.10,11 The currently approved Dresden protocol starts with epithelium removal followed by a 30-minute riboflavin soak before UVA irradiation for another 30 minutes (Figure 3).12
The pivotal trial for epithelium-off CXL in the US was a prospective, randomized, controlled clinical trial that examined 205 patients with progressive keratoconus.9 Patients were randomized into a treatment group, which underwent CXL with the Dresden protocol, and a sham control group, which received the photosensitizer but no epithelial debridement or UV exposure.12 The primary outcome was the mean change in maximum keratometry (Kmax) value at 12 months post treatment.9 At 12 months, the sham group steepened 1.0D on Kmax while the CXL group showed Kmax flattening of 1.6D.9
 |
|
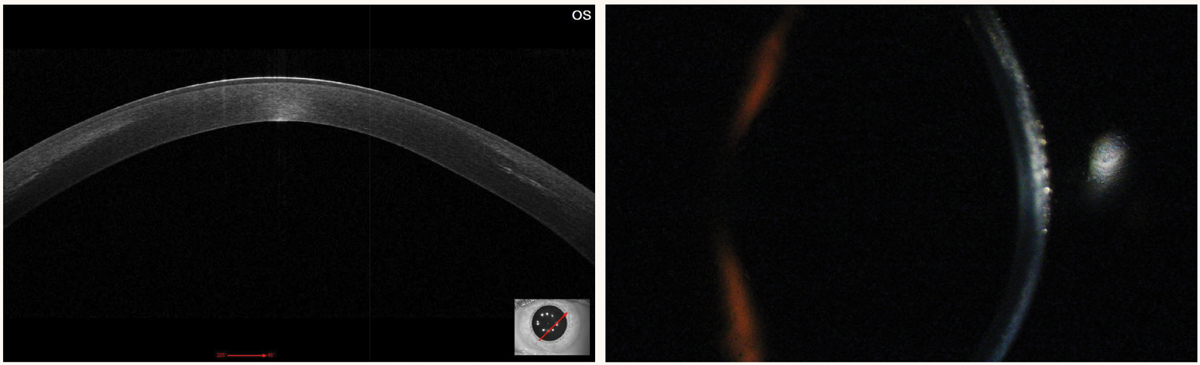
Fig. 5. Patient one month post-CXL with anterior corneal haze. Note the demarcation/hyperreflective line on OCT. Click image to enlarge. |
A crucial step in the CXL referral process is patient education and setting realistic expectations. Epithelium-off crosslinking is FDA approved and, when progression is well documented, commercial insurance coverage for patients is now quite good (Figure 4). Compared with 2017, when only three insurance carriers covered CXL, today 96% of commercially insured patients have coverage for CXL.13
Epithelium-off CXL’s primary goal is to freeze the cornea in place, and large improvements in corneal flattening or visual acuity can’t be over-promised.
Optometrists referring patients for CXL can help prepare patients by telling them two things: initially vision will be worse for about a week before it moves back to baseline, and the surgical eye will have a mild to moderate amount of pain, discomfort and photophobia for three to four days.
|
General Post-op Guidelines for Corneal Crosslinking* Day 1: This visit is optional for some providers • Assure bandage lens is in place • Discuss pain management and postoperative medications • Central epithelial defect will be present • Vision often significantly worse than pre-op Day 4-7: Main decision is whether to remove bandage lens • Epithelialization usually complete • Pain should be improving • Review postoperative medications • Vision still decreased from baseline, but functional Day 30: • Finished or finishing postoperative medications • Vision approaching or at baseline • Slit lamp shows early corneal haze • May release temporary spectacle prescription, if needed Day 90: • Corneal haze improving • May begin contact lens fitting • Release new spectacle Rx Annually: • Update topography/tomography to confirm stability
*May vary by provider and practice. |
Although pre-op CXL education should focus on the goal of stability, a clinical trial showed on average patients achieved Kmax flattening plus a bonus of mild improvement to BCVA.9 The most frequent adverse event in the treatment group was corneal haze, but only three eyes had retained haze/scarring at one year, and only two of those showed a decrease in BCVA.9 Long-term, CXL has shown continued corneal stability out to seven and 13 years in two separate studies.14,15 One study had a Kmax progression in 0% of cases, while the other had a 7% failure rate at 13 years.14,15
The future is also promising for accelerated epithelium-on CXL (ACXL) in the US. ACXL speeds up the procedure and healing for patients and may allow more patients to be treated. Two hurdles in the epithelium-on quest are the barrier function of the epithelium and the CXL reaction requirement of surplus oxygen.16 Epithelium-on CXL is not currently approved, but FDA trials are underway.
Optimizing post-op care for CXL requires a collaborative approach between the referring practitioner and the surgical practice. CXL has no global period, so follow-up care providers should bill office visits and ancillary testing accordingly. Similar to photorefractive keratectomy (PRK), an important early healing step is achieving corneal epithelialization and deciding when to remove the bandage contact lens.
Immediately following the procedure, we can educate patients that their vision will be worse, slowly improving to functional vision around one week and back to baseline at post-op month one to three. If a topography/tomography is performed at one- or three-months post-op, it is not uncommon to see steepening/worsening secondary to epithelial remodeling, and the most informative keratometry stability measurements are nine to 12 months postoperatively. Similarly, corneal pachymetry often measures thinner post-op. Stromal corneal haze is an expected finding at one- and three-months post-op and predictably will fade over time (Figure 5). This haze is visually insignificant and resolves without further intervention.17
 |
|
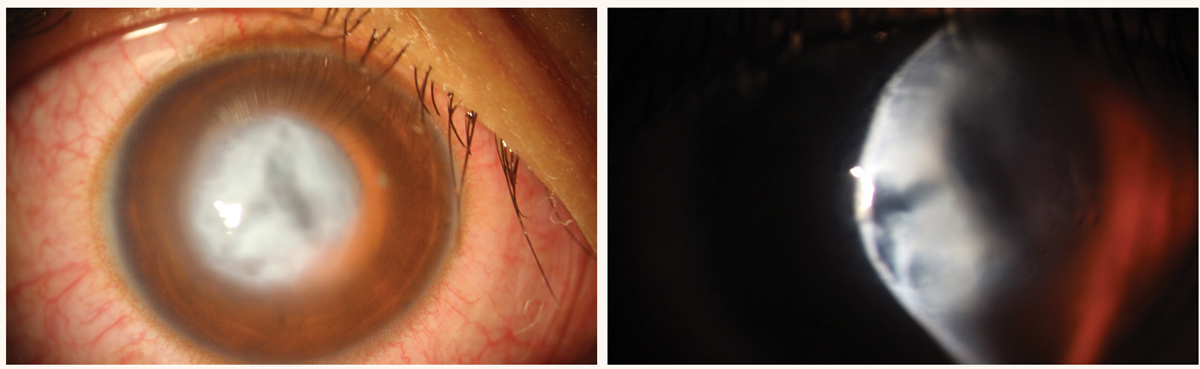
Fig. 6. Acute corneal hydrops in a patient with severe keratoconus. Click image to enlarge. |
Since CXL is not a refractive procedure, patients may be anxious for new glasses or contact lenses. Depending on patient function, the Rx can be predictably updated at three months post-op. For patients with exceptional needs, prescription or re-fitting may occur sooner than three months with the expectation that significant adjustments may be necessary in the near-term as the cornea stabilizes.
Vision Rehabilitation
The proposed third step in our new mantra for keratoconus management is vision rehabilitation, an area where optometrists should be front and center. Once the cornea is biomechanically, and largely refractively stabilized, a myriad of options opens up in the refractive toolbox. In reviewing these options, all have their individual “pros” and “cons,” and they are commonly employed together.
No matter the severity of the keratoconus, it is important that patients have an updated glasses prescription. At minimum glasses provide a “contact lens break” in patients who are heavily reliant on these lenses. If a patient’s keratoconus is mild and glasses correction satisfies their visual needs, this is a great option because patient convenience and safety is unmatched. Unfortunately, in moderate to severe ectatic disease where more corneal warpage has occurred, higher-order aberrations like glare, starbursts and ghosting aren’t corrected by spectacle lenses. If a keratoconus patient has developed ectasia-induced scarring—for example, a patient with a history of corneal hydrops (Figure 6)—glasses will be of little visual benefit.
 |
|
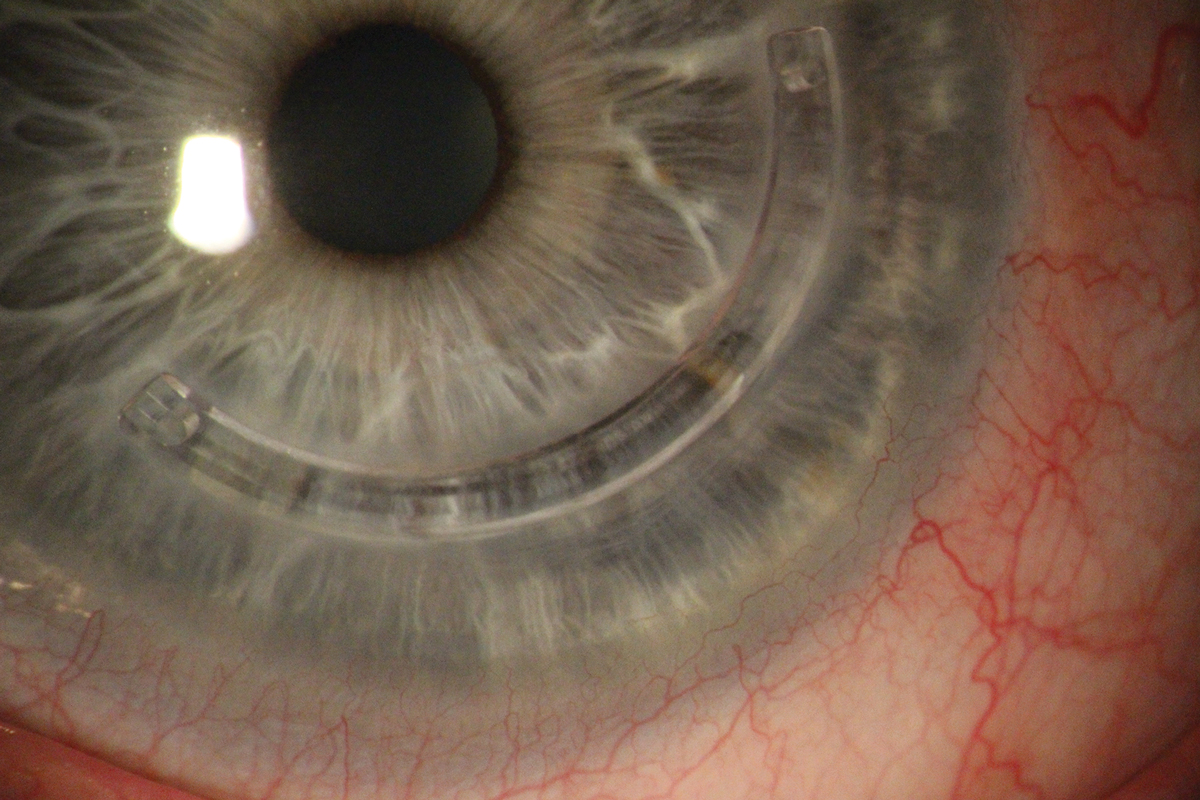
Fig. 7. Inferior Intacs ring placed in a patient with an inferiorly decentered cone. Click image to enlarge. |
Contact lenses for keratoconus can be divided into “soft disposable lenses” and “specialty hard lenses.” In large part, disposable soft contact lenses provide similar vision quality in ectatic corneas compared to glasses because the soft disposable lenses mold to the irregular anterior corneal shape. Specialty soft lenses, which are dispensed in more mild disease, are designed for the irregular cornea and feature an increased lens center thickness to help retain their shape over an irregular cornea thereby providing more optimal vision correction.
Gas permeable (GP) contact lens designs are more frequently used for patients spanning all levels of keratoconic disease because the tear prism under the lens masks the irregular anterior refractive surface. By minimizing higher order aberrations (glare, ghosting, starbursts) in ectatic corneas, the vision improvement with GP lenses can be life-changing for patients. There are a variety of gas permeable lens options available from smaller corneal and limbal designs to larger diameter scleral lenses. If the optics of a gas permeable lens are desirable, hybrid lenses (GP center and soft lens skirt) are also an option for the irregular cornea patient.
Specialty contact lenses not only improve patient visual function, but by masking corneal irregularities, they also likely delay or prevent corneal transplantation surgeries for patients.18-20 One of the newest studies compared the rates of keratoplasty in patients who wore either no lenses, soft lenses, corneal gas permeable lenses or scleral lenses for vision rehabilitation in keratoconus. This study concluded that GP corneal lens or scleral lens wear significantly lowered the risk of undergoing keratoplasty.20
In specialty lenses, specifically scleral lenses, innovations in lens Dk (oxygen permeability), solutions and coatings have improved patient comfort, wear time and negative visual symptoms. Increasing a lenses Dk in simple terms means increasing the oxygen transmissibility from the environment to the cornea. Studies have suggested increasing Dk to a value of 150 or higher, but physicians must balance higher Dk lenses with the potential for less wettability and increased lens debris deposition.21
Hydra-PEG (Tangible Science) is a novel biocompatible polymer that creates a more consistent and durable lens coating. Hydra-PEG surface treatment has been shown to increase lens comfort, decrease lens deposits and decrease lens fogging.22 A third expanding area is scleral lens insertion solutions. Buffered vs. non-buffered products, single-use vials and the addition of electrolytes among others have all been additions to practitioner options.
A final addition in scleral lenses is impression-fit lenses. Analogous to a dentist using impression molds to make dental implants with a precision fit, these impression-based scleral lenses can be customized to a patient’s cornea and sclera. Impression-based lenses can serve all types of patients, but commonly they are reserved for highly irregular corneas with more advanced pathology.23
In the surgical space for keratoconus vision rehab, there are both addition procedures and subtraction procedures. First, addition procedures revolve around using either biocompatible PMMA intrastromal ring segments (Figure 7) or allogenic ring segments inserted into the deep stroma.
Intrastromal ring segments aim to flatten and reshape the ectatic cornea while concurrently mechanically supporting the cornea. By mechanically centering the steep irregular cone, ICRS have the ability to decrease myopia and astigmatism, leading to better vision in glasses and disposable contact lenses. In our practice, our usage of Intacs most commonly is sequential to corneal crosslinking. When a patient’s best-corrected glasses vision is less than 20/40, or a patient is unable or unwilling to wear specialty contact lenses, these are the most frequent scenarios in which our team deploys Intacs.
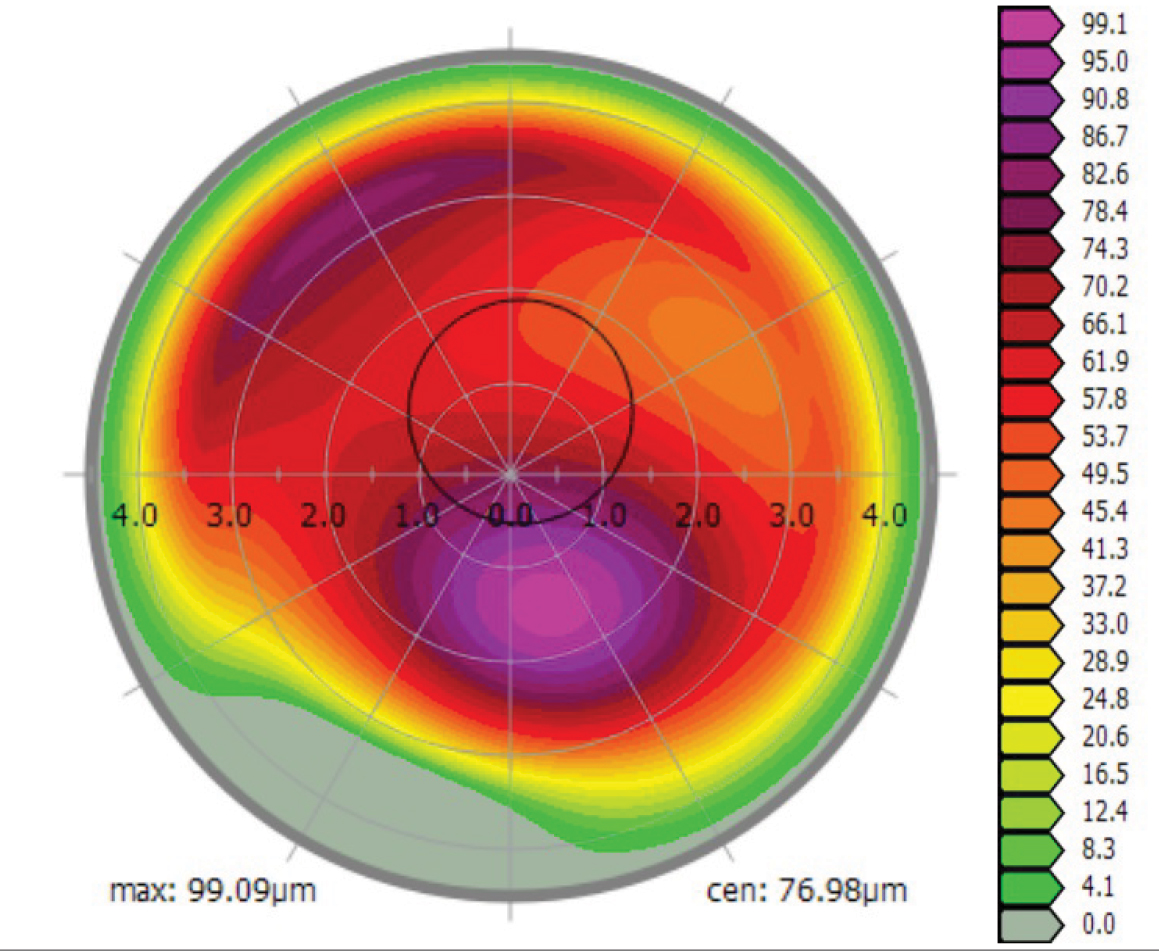
Finally, topography-guided PRK (TG-PRK) offers laser vision correction for keratoconus patients who have previously been told such procedures are contraindicated. Topography-guided treatments base the laser ablation profile on shape data from each patient’s specific corneal shape/topography. Topography-guided laser vision correction is FDA-approved for normal corneas, but some surgeons are using the technology off-label for highly irregular corneas. Two differentiators for TG-PRK treatments are, first, the Contoura (Alcon) placido disc topographer’s ability to measure highly aberrated corneas and plan a surgical treatment and, second, the platform’s ability to do more focal ablations. In keratoconus patients, this often means delivering more laser energy to the inferior steep cone and minimizing superior ablation energy, resulting in a regularization of the anterior corneal shape (Figure 8).
 |
|
Fig. 8. Topography-guided PRK ablation profile for a keratoconic patient 12 months post-CXL. Note the inferior increased amount of planned laser energy. Click image to enlarge. |
A key in setting expectations for TG-PRK in keratoconus patients is to change the definition of “success” from total glasses and contact lens independence to alleviating the dependence on specialty lenses, therefore gaining good visual quality in glasses and contact lenses.
Optometry’s role in the management of keratoconus is preserving patients’ visual potential and rehabilitating visual acuity to maintain a higher quality of life. Innovations in treatment allow practitioners to deviate from “monitoring the bend until it breaks,” to a more interventional mindset.
Researchers studied quality of life in keratoconus patients and, not surprisingly, BCVA in the better-seeing eye was the number one factor in quality of life.24 Early diagnosis and earlier intervention align beautifully with the goal of saving BCVA. CXL also contributes to higher quality of life scores in both early and late stages of keratoconus.24
Adopting a management approach where early diagnosis leads to intervention with CXL allows the practitioner to follow-up with a number of options for vision rehabilitation. This shifts patients away from a cycle of “watch and wait” and toward the new paradigm of “intervene and treat”—a more proactive approach that any eye care practitioner can support.
Dr. Ibach is a residency trained optometrist at Vance Thompson Vision in Sioux Falls, SD. He serves as an adjunct clinical faculty for the Illinois College of Optometry and the Pikesville College of Optometry. He receives consulting fees, fees for non-CME/CE services and conducts research with Glaukos.
1. Al-Mahrouqi H, Oraba S, Al-Habsi S, et al. Retinoscopy as a screening tool for keratoconus. Cornea, 2019;38(4):442-5. 2. Greenwald MF, Scruggs BA, Vislisel JM, et al. Corneal imaging: an introduction. EyeRounds.org. Posted October 19, 2016; Available from: EyeRounds.org/tutorials/corneal-imaging/index.htm. 3. Belin MW, Villavicencio OF, Ambrósio RR. (2014). Tomographic parameters for the detection of keratoconus: suggestions for screening and treatment parameters. Eye Contact Lens. 2014;40(6):326-30. 4. Pircher N, Schwarzhans F, Holzer S, et al. Distinguishing keratoconic eyes and healthy eyes using ultrahigh-resolution optical coherence tomography-based corneal epithelium thickness mapping. Am J Ophthalmol. 2018;189:47-54. 5. Wheeler J, Hauser MA, Afshari NA, et al. The genetics of keratoconus: a review. Reprod Syst Sex Disord. 2012;(Suppl 6):001. 6. Lapeyre G, Fournie P, Vernet R, et al. Keratoconus prevalence in families: a french study. Cornea. 2020;39(12):1473-9. 7. Trattler W, Kramer E. (2020, October). Identifying, treating, and monitoring the progression of keratoconus. Cataract and Refractive Surgery Today. 2020;20(10):35-6. 8. Ferdi A, Nguyen V, Gore D, et al. Keratoconus natural progression: a systematic review and meta-analysis of 11,529 Eyes. Ophthalmol. 2019;126(7):935-45. 9. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmol. 2017;124(9):1259-70. 10. Bradford SM, Mikula ER, Juhasz T, et al. Collagen fiber crimping following in vivo UVA-induced corneal crosslinking. Exp Eye Res. 2018;177:173-80. 11. Wollensak G, Iomdina E. Long‐term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmologica. 2009;87(1):48-51. 12. Belin M, Lim L, Rajpal R, Hafezi F, et al. Corneal Cross-Linking: current USA status: report from the cornea society. Cornea. 2018;37(10):1218-25. 13. Living with Keratoconus. www.livingwithkc.com. 14. Vinciguerra R, Pagano L, Borgia A, et al. Corneal cross-linking for progressive keratoconus: up to 13 years of follow-up. J Refract Surg. 2020;36(12), 838-43. 15. O’Brart D, Patel P, Lascaratos G, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. 2015;160(6):1154-63. 16. Hill J, Liu C, Deardorff P, et al. Optimization of oxygen dynamics, UV-A delivery, and drug formulation for accelerated epi-on corneal crosslinking. Curr Eye Res. 2020;45(4):450-8. 17. Greenstein SA, Fry KL, Bhatt J. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2010;36(12):2105-14. 18. DeLoss KS, Fatteh NH, Hood CT. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) scleral device compared to keratoplasty for the treatment of corneal ectasia. Am J Ophthalmol. 2014;158(5):974-82. 19. Koppen C, Kreps EO, Anthonissen L, et al Scleral Lenses Reduce the Need for Corneal Transplants in Severe Keratoconus. Am J Ophthalmol. 2018;185:43-47. 20. Ling J, Mian S, Stein J, et al. Impact of scleral contact lens use on the rate of corneal transplantation for keratoconus. Cornea. 2021;40(1):39-42. 21. Michaud L, van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012;5(6):266-71. 22. Mickles CV, Harthan JS, Barnett M. Assessment of a novel lens surface treatment for scleral lens wearers with dry eye. Eye Contact Lens. 2020; doi: 10.1097/ICL.0000000000000754. 23. Nau A, Shorter ES, Harthan JS, et al. Multicenter review of impression-based scleral devices. Cont Lens Anterior Eye. 2020:S1367-0484(20)30193-4. 24. Panthier C, Moran S, Bourges JL. Evaluation of vision-related quality of life in keratoconus patient, and associated impact of keratoconus severity indicators. Graefes Arch Clin Exp Ophth. 2020;258(7):1459-68. 25. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267-73. 26. Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263-70. 27. Chan E, Chong EW, Lingham G, et al. Prevalence of keratoconus based on Scheimpflug imaging: The Raine Study. Ophthalmology. 2020; S0161-6420(20)30838-1. 28. Nowak, D. M., & Gajecka, M. (2011). The genetics of keratoconus. Middle East Afr J Ophthalmol. 2011;18(1):2-6. 29. Wagner H, Barr J, Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-32. 30. Alio J, Vega-Estrada A, Sanz P, et al. Corneal morphologic characteristics in patients with down syndrome. JAMA Ophthalmol. 2018;136(9):971-8. |
